Temperature-Dependent Chirality in Halide Perovskites
- PMID: 39083667
- PMCID: PMC11318036
- DOI: 10.1021/acs.jpclett.4c01629
Temperature-Dependent Chirality in Halide Perovskites
Abstract
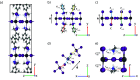
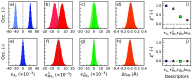
With the use of chiral organic cations in two-dimensional metal halide perovskites, chirality can be induced in the metal halide layers, which results in semiconductors with intriguing chiral optical and spin-selective transport properties. The chiral properties strongly depend upon the temperature, despite the basic crystal symmetry not changing fundamentally. We identify a set of descriptors that characterize the chirality of metal halide perovskites, such as MBA2PbI4, and study their temperature dependence using molecular dynamics simulations with on-the-fly machine-learning force fields obtained from density functional theory calculations. We find that, whereas the arrangement of organic cations remains chiral upon increasing the temperature, the inorganic framework loses this property more rapidly. We ascribe this to the breaking of hydrogen bonds that link the organic with the inorganic substructures, which leads to a loss of chirality transfer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Nespolo M.; Aroyo M. I.; Souvignier B. Crystallographic Shelves: Space-Group Hierarchy Explained. J. Appl. Crystallogr. 2018, 51, 1481–1491. 10.1107/S1600576718012724. - DOI
-
- Waldeck D. H.; Naaman R.; Paltiel Y. The Spin Selectivity Effect in Chiral Materials. APL Mater. 2021, 9, 040902. 10.1063/5.0049150. - DOI
LinkOut - more resources
Full Text Sources

