Efficacy and Safety of Sacituzumab Govitecan in Patients With Advanced Solid Tumors (TROPiCS-03): Analysis in Patients With Advanced Endometrial Cancer
- PMID: 39083724
- PMCID: PMC11458108
- DOI: 10.1200/JCO.23.02767
Efficacy and Safety of Sacituzumab Govitecan in Patients With Advanced Solid Tumors (TROPiCS-03): Analysis in Patients With Advanced Endometrial Cancer
Abstract
Purpose: Patients with advanced endometrial cancer (EC) who progress on or after platinum-based therapy and immunotherapy have poor prognosis. We report efficacy and safety of sacituzumab govitecan (SG), a trophoblast cell-surface antigen 2 (Trop-2)-directed antibody-drug conjugate, in patients with advanced EC.
Methods: TROPiCS-03 (ClinicalTrials.gov identifier: NCT03964727) is a multicohort, open-label, phase II basket study in patients with metastatic solid tumors. Eligible patients in the EC cohort received SG 10 mg/kg once on days 1 and 8 every 3 weeks. Primary end point was objective response rate (ORR) by investigator's assessment per RECIST v1.1. Secondary end points included clinical benefit rate (CBR; complete and partial response, and stable disease ≥6 months), duration of response (DOR), and progression-free survival (PFS) per investigator assessment, overall survival, and safety. Trop-2 expression of archival or baseline tumor specimens was analyzed by immunohistochemistry.
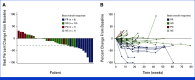
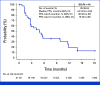
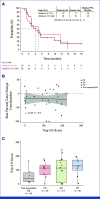
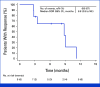
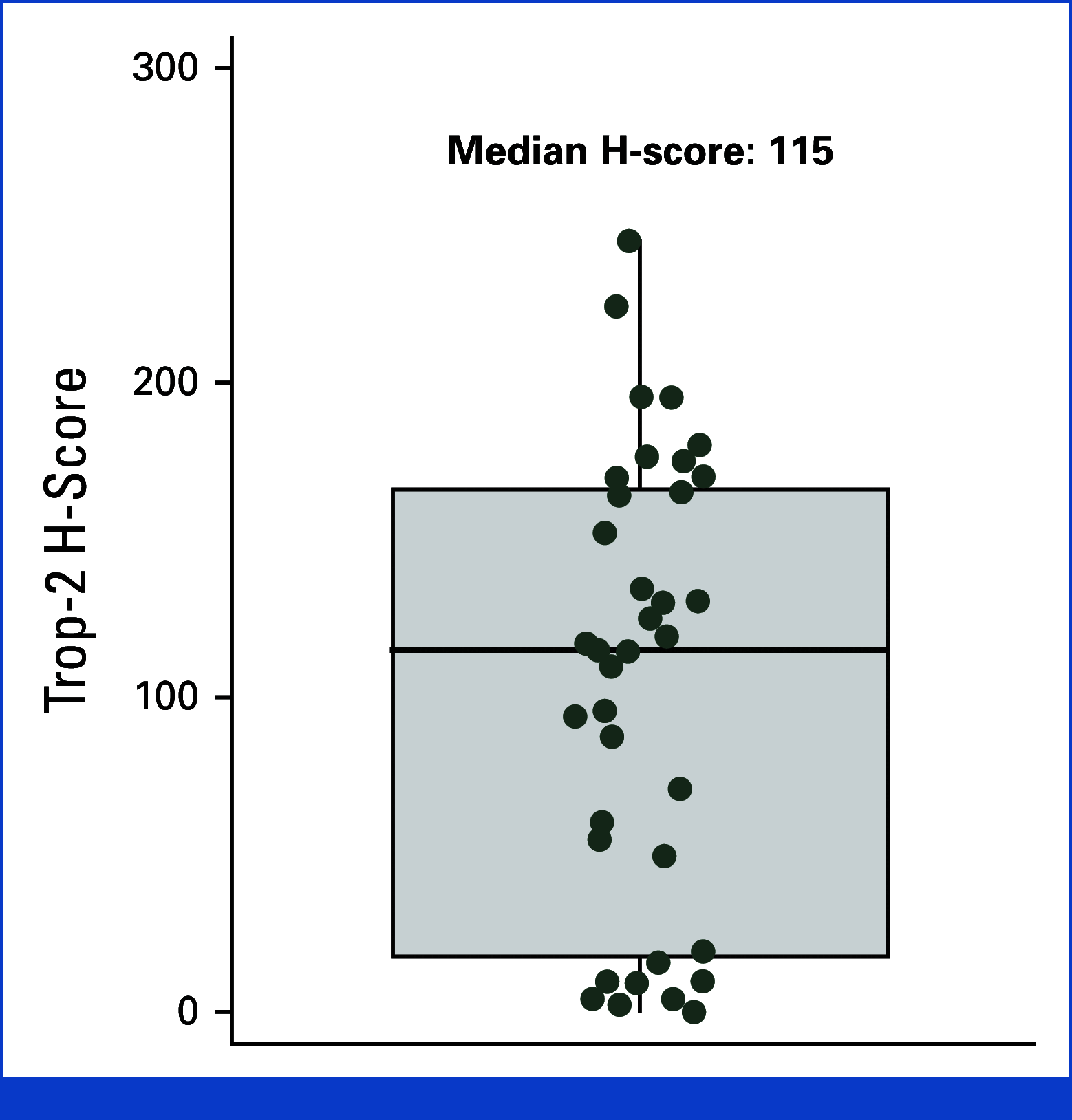
Results: At data extraction date, 41 patients were enrolled. Median follow-up was 5.8 months (range, 0.7-19.3); median previous therapies was three (range, 1-6); and 85% of patients received previous chemotherapy and immunotherapy. ORR was 22% (95% CI, 11 to 38); CBR was 32% (95% CI, 18 to 48). Median DOR was 8.8 months (95% CI, 2.8 to not estimable); median PFS was 4.8 months (95% CI, 2.8 to 9.8). Trop-2 exploratory analysis was conducted retrospectively for 39 patients. Tumor Trop-2 protein was highly expressed in EC, showing limited correlation with efficacy. Grade ≥3 treatment-related adverse events (TRAEs) occurred in 73% of patients. Study drug discontinuation due to TRAEs was 5%. Two deaths occurred, deemed unrelated to SG.
Conclusion: Findings from TROPiCS-03 showed encouraging efficacy of SG with a manageable toxicity profile in a heavily pretreated population with advanced EC. Safety findings were consistent with the known SG safety profile.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- International World Cancer Research Fund: Endometrial cancer statistics, 2022 . https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/
-
- Post CCB, Westermann AM, Boere IA, et al. : Efficacy and safety of durvalumab with olaparib in metastatic or recurrent endometrial cancer (phase II DOMEC trial). Gynecol Oncol 165:223-229, 2022 - PubMed
-
- Oaknin A, Bosse TJ, Creutzberg CL, et al. : Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:860-877, 2022 - PubMed
-
- National Comprehensive Cancer Network: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Uterine Neoplasms V.2.2024. National Comprehensive Cancer Network, Inc, 2024.
-
- Mirza MR, Chase DM, Slomovitz BM, et al. : Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 388:2145-2158, 2023 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

