Neutralizing Autoantibodies against Interleukin-10 in Inflammatory Bowel Disease
- PMID: 39083772
- PMCID: PMC7616361
- DOI: 10.1056/NEJMoa2312302
Neutralizing Autoantibodies against Interleukin-10 in Inflammatory Bowel Disease
Abstract
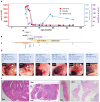
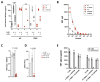
We discovered high-titer neutralizing autoantibodies against interleukin-10 in a child with infantile-onset inflammatory bowel disease (IBD), a phenocopy of inborn errors of interleukin-10 signaling. After B-cell-depletion therapy and an associated decrease in the anti-interleukin-10 titer, conventional IBD therapy could be withdrawn. A second child with neutralizing anti-interleukin-10 autoantibodies had a milder course of IBD and has been treated without B-cell depletion. We conclude that neutralizing anti-interleukin-10 autoantibodies may be a causative or modifying factor in IBD, with potential implications for therapy. (Funded by the National Institute for Health and Care Research and others.).
Copyright © 2024 Massachusetts Medical Society.
Figures
References
-
- Glocker E-O, Frede N, Perro M, et al. Infant colitis—it’s in the genes. The Lancet. 2010;376(9748):1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
