Atrial arrhythmia in adults with sickle cell anemia: a missing link toward understanding and preventing strokes
- PMID: 39083808
- PMCID: PMC11550361
- DOI: 10.1182/bloodadvances.2024013208
Atrial arrhythmia in adults with sickle cell anemia: a missing link toward understanding and preventing strokes
Abstract
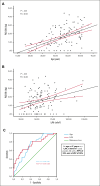
Although patients with homozygous sickle cell anemia (SCA) carry both significant left atrial (LA) remodeling and an increased risk of stroke, the prevalence of atrial arrhythmia (AA) has never been prospectively evaluated. The aim of this study was to identify the prevalence and predictors of atrial arrhythmia in SCA. From 2018 to 2022, consecutive adult patients with SCA were included in the DREPACOEUR prospective registry and referred to the physiology department for cardiac evaluation, including a 24-hour electrocardiogram monitoring (ECG-Holter). The primary endpoint was the occurrence of AA, defined by the presence of excessive supraventricular ectopic activity (ESVEA) on ECG-Holter (ie >720 premature atrial contractions [PACs] or any run ≥ 20 PACs) or any recent history of atrial fibrillation. Overall, 130 patients with SCA (mean age: 45±12 years, 48% of male) were included. AA was found in 34 (26%) patients. Age (52±9 vs. 42±12 years, P=0,002), LA dilation (LAVi, 71±24 vs. 52±14 mL/m², P<0.001) and history of stroke without underlying cerebral vasculopathy (26% vs. 5%, P=0.009, OR=6.6 (95%CI 1.4-30.3]) were independently associated with AA. Age and LAVi correlated with PAC load per 24 hours on ECG-Holter. An age over 47 years or a LAVi >55mL/m² could predict AA with a PPV of 33% and a NPV of 92%. AAs are frequent in middle-aged patients with SCA and increase with age and LA remodeling, leading to a major additional risk factor for ischemic stroke. This study provides arguments and means to early screen for AA and potentially prevent cerebral complications.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P. Bartolucci. received grants from Addmedica, Fabre Foundation, Novartis, and Bluebird in the past 36 months; consulting fees from Addmedica, Novartis, Roche, GBT, Bluebird, Emmaus, Hemanext, and Agios; received honoraria for lectures from Novartis, Addmedica, Jazz Pharmaceuticals; and reports being a member of the Novartis steering committee and cofounder of Innovhem. The remaining authors declare no competing financial interests.
Figures


References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl. 2016 Oct 8;388(10053):1545–1602. - PMC - PubMed
-
- Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. - PubMed
-
- Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387(10037):2565–2574. - PubMed
-
- d’Humières T, Savale L, Inamo J, et al. Cardiovascular phenotypes predict clinical outcomes in sickle cell disease: an echocardiography-based cluster analysis. Am J Hematol. 2021;96(9):1166–1175. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

