Botensilimab, an Fc-Enhanced Anti-CTLA-4 Antibody, Is Effective against Tumors Poorly Responsive to Conventional Immunotherapy
- PMID: 39083809
- PMCID: PMC11609826
- DOI: 10.1158/2159-8290.CD-24-0190
Botensilimab, an Fc-Enhanced Anti-CTLA-4 Antibody, Is Effective against Tumors Poorly Responsive to Conventional Immunotherapy
Abstract
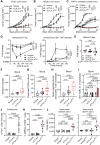
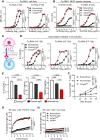
This study reveals that Fc-enhanced anti-CTLA-4 harnesses novel mechanisms to overcome the limitations of conventional anti-CTLA-4, effectively treating poorly immunogenic and treatment-refractory cancers. Our findings support the development of a new class of immuno-oncology agents, capable of extending clinical benefit to patients with cancers resistant to current immunotherapies.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
C. Delepine reports employment with Agenus Inc and ownership of stocks of the company. A. Tanne reports employment with and being a share holder of Agenus Inc at the time he worked on this project. H. Han reports grants from Agenus Inc during the conduct of the study. S. Bourdelais reports other support from Agenus Inc outside the submitted work. J.D. Waight reports personal fees from Agenus Inc during the conduct of the study. A. Sebastin-Yague reports personal fees from Agenus Inc and MiNK Therapeutics outside the submitted work. A. Slee reports employment with Agenus Inc. J.E. Grossman reports other support from Agenus Inc during the conduct of the study. N.S. Wilson reports a patent for
Figures




References
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541–7. - PubMed
-
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. . Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270:985–8. - PubMed
-
- Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, et al. . B70 antigen is a second ligand for CTLA-4 and CD28. Nature 1993;366:76–9. - PubMed
-
- Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science 1993;262:905–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

