Conditional loss of Brca1 in oocytes causes reduced litter size, ovarian reserve depletion and impaired oocyte in vitro maturation with advanced reproductive age in mice
- PMID: 39084071
- PMCID: PMC11342213
- DOI: 10.1016/j.ebiom.2024.105262
Conditional loss of Brca1 in oocytes causes reduced litter size, ovarian reserve depletion and impaired oocyte in vitro maturation with advanced reproductive age in mice
Abstract
Background: An estimated 1 in 350 women carry germline BRCA1/2 mutations, which confer an increased risk of developing breast and ovarian cancer, and may also contribute to subfertility. All mature, sex steroid-producing ovarian follicles are drawn from the pool of non-renewable primordial follicles, termed the 'ovarian reserve'. The clinical implications of early ovarian reserve exhaustion extend beyond infertility, to include the long-term adverse health consequences of loss of endocrine function and premature menopause. We aimed to determine whether conditional loss of Brca1 in oocytes impacts ovarian follicle numbers, oocyte quality and fertility in mice with advancing maternal age. We also aimed to determine the utility of AMH as a marker of ovarian function, by assessing circulating AMH levels in mice and women with BRCA1/2 mutations, and correlating this with ovarian follicle counts.
Methods: In this study, we addressed a longstanding question in the field regarding the functional consequences of BRCA1 inactivation in oocytes. To recapitulate loss of BRCA1 protein function in oocytes, we generated mice with conditional gene deletion of Brca1 in oocytes using Gdf9-Cre recombinase (WT: Brca1fl/flGdf9+/+; cKO: Brca1fl/flGdf9cre/+).
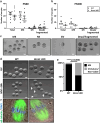
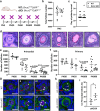
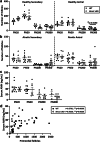
Findings: While the length of the fertile lifespan was not altered between groups after a comprehensive breeding trial, conditional loss of Brca1 in oocytes led to reduced litter size in female mice. Brca1 cKO animals had a reduced ovarian reserve and oocyte maturation was impaired with advanced maternal age at postnatal day (PN)300, compared to WT animals. Serum anti-Müllerian hormone (AMH) concentrations (the gold-standard indirect marker of the ovarian reserve used in clinical practice) were not predictive of reduced primordial follicle number in Brca1 cKO mice versus WT. Furthermore, we found no correlation between follicle number or density and serum AMH concentrations in matched samples from a small cohort of premenopausal women with BRCA1/2 mutations.
Interpretation: Together, our data demonstrate that BRCA1 is a key regulator of oocyte number and quality in females and suggest that caution should be used in relying on AMH as a reliable marker of the ovarian reserve in this context.
Funding: This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. This work was supported by funding from the Australian Research Council (ALW - DE21010037 and KJH - FT190100265), as well as the National Breast Cancer Foundation (IIRS-22-092) awarded to ALW and KJH. LRA, YML, LT, EOKS and MG were supported by Australian Government Research Training Program Scholarships. LRA, YML and LT were also supported by a Monash Graduate Excellence Scholarship. YC, SG and XC were supported by Monash Biomedicine Discovery Institute PhD Scholarships. LRA was also supported by a Monash University ECPF24-6809920940 Fellowship. JMS was supported by NHMRC funding (2011299). MH was supported by an NHMRC Investigator Grant (1193838).
Keywords: AMH; Ageing; BRCA1; DNA repair; Oocyte; Primordial follicle.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests LG is a consumer advocate for Breast Cancer Trials (BCT), a consumer representative for Breast Cancer Network Australia (BCNA), a patient partner for Breast International Group (BIG), and a patient advocate for Roche (Roche Holding AG). All other authors declare no competing financial, or other interests.
Figures


References
-
- McGee E.A., Hsueh A.J. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. - PubMed
-
- Findlay J.K., Hutt K.J., Hickey M., Anderson R.A. How is the number of primordial follicles in the ovarian reserve established? Biol Reprod. 2015;93(5):111. - PubMed
-
- Winship A.L., Stringer J.M., Liew S.H., Hutt K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update. 2018;24(2):119–134. - PubMed
-
- Morgan S., Anderson R.A., Gourley C., Wallace W.H., Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18(5):525–535. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

