Development of a functional beige fat cell line uncovers independent subclasses of cells expressing UCP1 and the futile creatine cycle
- PMID: 39084217
- PMCID: PMC12005060
- DOI: 10.1016/j.cmet.2024.07.002
Development of a functional beige fat cell line uncovers independent subclasses of cells expressing UCP1 and the futile creatine cycle
Abstract
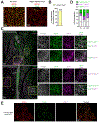
Although uncoupling protein 1 (UCP1) is established as a major contributor to adipose thermogenesis, recent data have illustrated an important role for alternative pathways, particularly the futile creatine cycle (FCC). How these pathways co-exist in cells and tissues has not been explored. Beige cell adipogenesis occurs in vivo but has been difficult to model in vitro; here, we describe the development of a murine beige cell line that executes a robust respiratory response, including uncoupled respiration and the FCC. The key FCC enzyme, tissue-nonspecific alkaline phosphatase (TNAP), is localized almost exclusively to mitochondria in these cells. Surprisingly, single-cell cloning from this cell line shows that cells with the highest levels of UCP1 express little TNAP, and cells with the highest expression of TNAP express little UCP1. Immunofluorescence analysis of subcutaneous fat from cold-exposed mice confirms that the highest levels of these critical thermogenic components are expressed in distinct fat cell populations.
Keywords: Alpl gene; SV40 large T antigen; TNAP; UCP1; aP2-Prdm16 transgenic mice; functional beige cell line; futile creatine cycle; immortalized beige adipocytes; thermogenesis.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
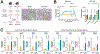


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

