A preclinical model of severe NASH-like liver injury by chronic administration of a high-fat and high-sucrose diet in mice
- PMID: 39084266
- PMCID: PMC11711102
- DOI: 10.1016/j.taap.2024.117046
A preclinical model of severe NASH-like liver injury by chronic administration of a high-fat and high-sucrose diet in mice
Abstract
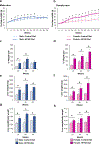
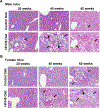
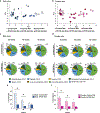
Non-alcoholic fatty liver disease (NAFLD) is a progressive liver disease, affecting 38% of adults globally. If left untreated, NAFLD may progress to more advanced forms of the disease, including non-alcoholic steatohepatitis (NASH), liver cirrhosis, and fibrosis. Early NAFLD detection is critical to prevent disease progression. Using an obesogenic high-fat and high-sucrose (HF/HS) diet, we characterized the progression of NAFLD in male and female Collaborative Cross CC042 mice after 20-, 40-, and 60-week intervals of chronic HF/HS diet feeding. The incidence and severity of liver steatosis, inflammation, and fibrosis increased in both sexes over time, with male mice progressing to a NASH-like disease state faster than female mice, as indicated by earlier and more pronounced changes in liver steatosis. Histopathological indication of macrovesicular steatosis and gene expression changes of key lipid metabolism genes were found to be elevated in both sexes after 20 weeks of HF/HS diet. Measurement of circulating markers of inflammation (CXCL10 and TNF-α), histopathological analysis of immune cell infiltrates, and gene expression changes in inflammation-related genes indicated significant liver inflammation after 40 and 60 weeks of HF/HS diet exposure in both sexes. Liver fibrosis, as assessed by Picosirius red and Masson's trichrome staining and changes in expression of key fibrosis related genes indicated significant changes after 40 and 60 weeks of HF/HS diet exposure. In conclusion, we present a preclinical animal model of dietary NAFLD progression, which recapitulates human pathophysiological and pathomorphological changes, that could be used to better understand the progression of NAFLD and support development of new therapeutics.
Keywords: Collaborative cross; Non-alcoholic fatty liver disease (NAFLD); Non-alcoholic steatohepatitis (NASH); Obesogenic diet; Preclinical mouse model.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen A-A The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol, 7: 851–861 (2022). - PubMed
-
- Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, Tan DJH, Tang ASP, Tay P, Xiao J, Yong JN, Zeng RW, Chew NWS, Nah B, Kulkarni A, Siddiqui MS, Dan YY, Wong VW-S, Sanyal AJ, Noureddin M, Muthiah M, Ng CH Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol, 8: 20–30 (2023). - PubMed
-
- Loomba R, Friedman SL, Shulman GI Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell, 184: 2537–2564 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

