Mucosal Single-Cell Profiling of Crohn's-Like Disease of the Pouch Reveals Unique Pathogenesis and Therapeutic Targets
- PMID: 39084267
- PMCID: PMC11973979
- DOI: 10.1053/j.gastro.2024.07.025
Mucosal Single-Cell Profiling of Crohn's-Like Disease of the Pouch Reveals Unique Pathogenesis and Therapeutic Targets
Abstract
Background & aims: The pathophysiology of Crohn's-like disease of the pouch (CDP) in patients with a history of ulcerative colitis (UC) is unknown. We examined mucosal cells from patients with and without CDP using single-cell analyses.
Methods: Endoscopic samples were collected from pouch body and prepouch ileum (pouch/ileum) of 50 patients with an ileal pouch-anal anastomosis. Single-cell RNA sequencing was performed on pouch/ileal tissues of patients with normal pouch/ileum and CDP. Mass cytometry was performed on mucosal immune cells from patients with UC with normal pouch/ileum, CDP, pouchitis, and those with familial adenomatous polyposis after pouch formation. Findings were independently validated using immunohistochemistry.
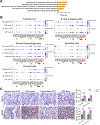
Results: The cell populations/states in the pouch body differed from those in the prepouch ileum, likely secondary to increased microbial burden. Compared with the familial adenomatous polyposis pouch, the UC pouch was enriched in colitogenic immune cells even without inflammation. CDP was characterized by increases in T helper 17 cells, inflammatory fibroblasts, inflammatory monocytes, TREM1+ monocytes, clonal expansion of effector T cells, and overexpression of T helper 17 cells-inducing cytokine genes such as IL23, IL1B, and IL6 by mononuclear phagocytes. Ligand-receptor analysis further revealed a stromal-mononuclear phagocytes-lymphocyte circuit in CDP. Integrated analysis showed that up-regulated immune mediators in CDP were similar to those in CD and pouchitis, but not UC. Additionally, CDP pouch/ileum exhibited heightened endoplasmic reticulum stress across all major cell compartments.
Conclusions: CDP likely represents a distinct entity of inflammatory bowel disease with heightened endoplasmic reticulum stress in both immune and nonimmune cells, which may become a novel diagnostic biomarker and therapeutic target for CDP.
Keywords: CyTOF; IBD; IPAA; Pouch; scRNA-seq.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures






References
-
- Shen B, Kochhar GS, Kariv R, et al. Diagnosis and classification of ileal pouch disorders: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol 2021;6:826–849. - PubMed
-
- Shen B, Kochhar GS, Rubin DT, et al. Treatment of pouchitis, Crohn’s disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol 2022;7:69–95. - PubMed
-
- Cao SY, Cella M, Jaeger N, et al. Mass cytometry analysis of Crohn’s disease-like phenotype of the ileoanal pouch following ileal pouch-anal anastomosis for ulcerative colitis. Gastroenterology 2022;162:S58–S59.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

