Autoregulatory mechanism of enzyme activity by the nuclear localization signal of lysine-specific demethylase 1
- PMID: 39084460
- PMCID: PMC11388019
- DOI: 10.1016/j.jbc.2024.107607
Autoregulatory mechanism of enzyme activity by the nuclear localization signal of lysine-specific demethylase 1
Abstract
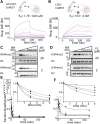
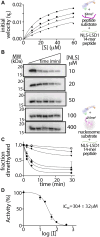
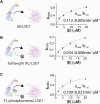
The N-terminal region of the human lysine-specific demethylase 1 (LSD1) has no predicted structural elements, contains a nuclear localization signal (NLS), undergoes multiple posttranslational modifications (PTMs), and acts as a protein-protein interaction hub. This intrinsically disordered region (IDR) extends from core LSD1 structure, resides atop the catalytic active site, and is known to be dispensable for catalysis. Here, we show differential nucleosome binding between the full-length and an N terminus deleted LSD1 and identify that a conserved NLS and PTM containing element of the N terminus contains an alpha helical structure, and that this conserved element impacts demethylation. Enzyme assays reveal that LSD1's own electropositive NLS amino acids 107 to 120 inhibit demethylation activity on a model histone 3 lysine 4 dimethyl (H3K4me2) peptide (Kiapp ∼ 3.3 μM) and histone 3 lysine 4 dimethyl nucleosome substrates (IC50 ∼ 30.4 μM), likely mimicking the histone H3 tail. Further, when the identical, inhibitory NLS region contains phosphomimetic modifications, inhibition is partially relieved. Based upon these results and biophysical data, a regulatory mechanism for the LSD1-catalyzed demethylation reaction is proposed whereby NLS-mediated autoinhibition can occur through electrostatic interactions, and be partially relieved through phosphorylation that occurs proximal to the NLS. Taken together, the results highlight a dynamic and synergistic role for PTMs, intrinsically disordered regions, and structured regions near LSD1 active site and introduces the notion that phosphorylated mediated NLS regions can function to fine-tune chromatin modifying enzyme activity.
Keywords: NMR; autoinhibition; histone demethylase; inhibition mechanism; intrinsically disordered protein; nuclear localization signal (NLS); nucleosome; structural biology.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Marabelli C., Marrocco B., Mattevi A. The growing structural and functional complexity of the LSD1/KDM1A histone demethylase. Curr. Opin. Struct. Biol. 2016;41:135–144. - PubMed
-
- Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

