Metabolic control of collagen synthesis
- PMID: 39084474
- PMCID: PMC11402592
- DOI: 10.1016/j.matbio.2024.07.003
Metabolic control of collagen synthesis
Abstract
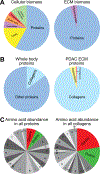
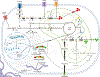
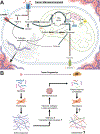
The extracellular matrix (ECM) is present in all tissues and crucial in maintaining normal tissue homeostasis and function. Defects in ECM synthesis and remodeling can lead to various diseases, while overproduction of ECM components can cause severe conditions like organ fibrosis and influence cancer progression and therapy resistance. Collagens are the most abundant core ECM proteins in physiological and pathological conditions and are predominantly synthesized by fibroblasts. Previous efforts to target aberrant collagen synthesis in fibroblasts by inhibiting pro-fibrotic signaling cascades have been ineffective. More recently, metabolic rewiring downstream of pro-fibrotic signaling has emerged as a critical regulator of collagen synthesis in fibroblasts. Here, we propose that targeting the metabolic pathways involved in ECM biomass generation provides a novel avenue for treating conditions characterized by excessive collagen accumulation. This review summarizes the unique metabolic challenges collagen synthesis imposes on fibroblasts and discusses how underlying metabolic networks could be exploited to create therapeutic opportunities in cancer and fibrotic disease. Finally, we provide a perspective on open questions in the field and how conceptual and technical advances will help address them to unlock novel metabolic vulnerabilities of collagen synthesis in fibroblasts and beyond.
Keywords: Cancer; Collagen; ECM; Fibroblasts; Fibrosis; Glycine; Metabolism; Nutrients; Proline.
Copyright © 2024 Elsevier B.V. All rights reserved.
Figures
References
-
- McAndrews KM, Miyake T, Ehsanipour EA, Kelly PJ, Becker LM, McGrail DJ, Sugimoto H, LeBleu VS, Ge Y, Kalluri R, Dermal αSMA+ myofibroblasts orchestrate skin wound repair via β1 integrin and independent of type I collagen production, The EMBO Journal 41 (2022) e109470. 10.15252/embj.2021109470. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

