Elevated antibody binding to striatal cholinergic interneurons in patients with pediatric acute-onset neuropsychiatric syndrome
- PMID: 39084540
- PMCID: PMC11569416
- DOI: 10.1016/j.bbi.2024.07.044
Elevated antibody binding to striatal cholinergic interneurons in patients with pediatric acute-onset neuropsychiatric syndrome
Abstract
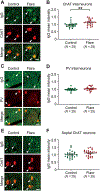
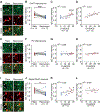
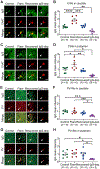
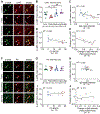
Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) is characterized by the abrupt onset of significant obsessive-compulsive symptoms (OCS) and/or severe food restriction, together with other neuropsychiatric manifestations. An autoimmune pathogenesis triggered by infection has been proposed for at least a subset of PANS. The older diagnosis of Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus (PANDAS) describes rapid onset of OCD and/or tics associated with infection with Group A Streptococcus. The pathophysiology of PANS and PANDAS remains incompletely understood. We recently found serum antibodies from children with rigorously defined PANDAS to selectively bind to cholinergic interneurons (CINs) in the striatum. Here we examine this binding in children with relapsing and remitting PANS, a more heterogeneous condition, collected in a distinct clinical context from those examined in our previous work, from children with a clinical history of Streptococcus infection. IgG from PANS cases showed elevated binding to striatal CINs in both mouse and human brain. Patient plasma collected during symptom flare decreased a molecular marker of CIN activity, phospho-riboprotein S6, in ex vivo brain slices; control plasma did not. Neither elevated antibody binding to CINs nor diminished CIN activity was seen with plasma collected from the same children during remission. These findings replicate what we have seen previously in PANDAS and support the hypothesis that at least a subset of PANS cases have a neuroimmune pathogenesis. Given the critical role of CINs in modulating basal ganglia function, these findings confirm striatal CINs as a locus of interest in the pathophysiology of both PANS and PANDAS.
Keywords: Antibody binding; Cholinergic interneurons; Dorsal striatum; Neuronal activity; OCD; PANDAS; PANS; Phospho-rpS6; Plasma autoantibodies.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Pittenger has served as a consultant and received research funding in the past year for Biohaven Pharmaceuticals, Transcend Therapeutics, Ceruvia Lifesciecnes, Freedom Biosciences, and Nobilis Therapeutics, and royalties from Oxford University Press, all for work unrelated to the current results. He is an inventor on a patent applications related to the use of neurofeedback and of psychedelic drugs for the treatment of OCD, also unrelated to this work. Dr. Che has received research funding from Duraviva Pharma for work unrealted to the current results. All other authors report no competing interests. The authors have declared that no conflict of interest exists.
Figures
References
-
- Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, Bossuyt X, Musset L, Cervera R, Plaza-Lopez A, Dias C, Sousa MJ, Radice A, Eriksson C, Hultgren O, Viander M, Khamashta M, Regenass S, Andrade LE, Wiik A, Tincani A, Rönnelid J, Bloch DB, Fritzler MJ, Chan EK, Garcia-De La Torre I, Konstantinov KN, Lahita R, Wilson M, Vainio O, Fabien N, Sinico RA, Meroni P, Shoenfeld Y, 2014. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 73, 17–23. doi: 10.1136/annrheumdis-2013-203863 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

