Real-world retrospective analysis of immune checkpoint inhibitor therapy for relapsed or refractory Hodgkin's lymphoma
- PMID: 39085129
- PMCID: PMC11528253
- DOI: 10.3960/jslrt.24021
Real-world retrospective analysis of immune checkpoint inhibitor therapy for relapsed or refractory Hodgkin's lymphoma
Abstract
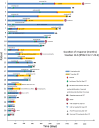
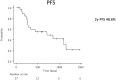
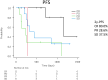
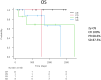
Immune checkpoint inhibitors (ICI) are promising therapeutic agents for relapsed or refractory classical Hodgkin's lymphoma (RRcHL). This retrospective study evaluated patients with RRcHL registered in the clinical research program Tohoku-Hematology-Forum-26, between 2016 and 2020, and treated with ICI in 14 centers in Northeast Japan. We analyzed the usage, efficacy, and safety of ICI therapy (ICIT). Among a total of 27 patients with RRcHL, 21 and nine were treated with nivolumab and/or pembrolizumab, respectively. The best response was complete response (CR), partial response (PR), stable disease (SD), and progressive disease in 11 (40.8%), seven (25.9%), eight (29.6%), and one (3.7%) patient, respectively. In all patients undergoing ICIT, the 2-year progression-free survival and 2-year overall survival (OS) were 48.6% and 87.4%, respectively. The 2-year OS for patients with CR, PR, and SD were 100%, 68.6%, and 87.5%, respectively. A total of 36 events of immune-related adverse events (irAEs) or immune-related like adverse events (irlAEs) were observed in 19 of the 27 patients (70.4%). Two thirds of these irAEs or irlAEs were grade 1-2 and controllable. During the observation period, ICIT was discontinued in 22 of 27 (81.4%) patients due to CR, inadequate response, irAE and patient circumstances in five (22.7%), seven (31.8%), eight (36.4%) and two patients (9.1%), respectively. Therapy-related mortality-associated irAE were observed in only one patient during ICIT. These results suggest that ICIT for RRcHL is effective and safe in real-world settings. The optimal timing of induction and duration of ICIT remains to be established.
Keywords: classical Hodgkin’s lymphoma; immune checkpoint inhibitors; real-world setting; relapsing and refractory cHL.
Conflict of interest statement
CONFLICT OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. Inst = My Institution. Relationships may not relate to the subject matter of this manuscript.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394-424. - PubMed
-
- Lymphoma Study Group of Japanese Pathologists . The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Pathol Int. 2000; 50: 696-702. - PubMed
-
- Nagai H. JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-10. Hodgkin lymphoma (HL). Int J Hematol. 2020; 111: 166-179. - PubMed
-
- Straus DJ, Długosz-Danecka M, Connors JM, et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021; 8: e410-e421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

