Depression clinical trials worldwide: a systematic analysis of the ICTRP and comparison with ClinicalTrials.gov
- PMID: 39085220
- PMCID: PMC11291508
- DOI: 10.1038/s41398-024-03031-6
Depression clinical trials worldwide: a systematic analysis of the ICTRP and comparison with ClinicalTrials.gov
Abstract
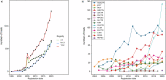
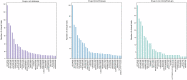
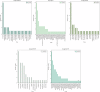
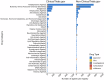
Major depressive disorder (MDD), commonly known as depression, affects over 300 million people worldwide as of 2018 and presents a wide range of clinical symptoms. The international clinical trials registry platform (ICTRP) introduced by WHO includes aggregated data from ClinicalTrials.gov and 17 other national registers, making it the largest clinical trial platform. Here we analysed data in ICTRP with the aim of providing comprehensive insights into clinical trials on depression. Applying a novel hidden duplicate identification method, 10,606 depression trials were identified in ICTRP, with ANZCTR being the largest non- ClinicalTrials.gov database at 1031 trials, followed by IRCT with 576 trials, ISRCTN with 501 trials, CHiCTR with 489 trials, and EUCTR with 351 trials. The top four most studied drugs, ketamine, sertraline, duloxetine, and fluoxetine, were consistent in both groups, but ClinicalTrials.gov had more trials for each drug compared to the non-ClinicalTrials.gov group. Out of 9229 interventional trials, 663 unique agents were identified, including approved drugs (74.5%), investigational drugs (23.2%), withdrawn drugs (1.8%), nutraceuticals (0.3%), and illicit substances (0.2%). Both ClinicalTrials.gov and non-ClinicalTrials.gov databases revealed that the largest categories were antidepressive agents (1172 in ClinicalTrials.gov and 659 in non-ClinicalTrials.gov) and nutrients, amino acids, and chemical elements (250 in ClinicalTrials.gov and 659 in non-ClinicalTrials.gov), indicating a focus on alternative treatments involving dietary supplements and nutrients. Additionally, 26 investigational antidepressive agents targeting 16 different drug targets were identified, with buprenorphine (opioid agonist), saredutant (NK2 antagonist), and seltorexant (OX2 antagonist) being the most frequently studied. This analysis addresses 40 approved drugs for depression treatment including new drug classes like GABA modulators and NMDA antagonists that are offering new prospects for treating MDD, including drug-resistant depression and postpartum depression subtypes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The international clinical trials registry platform (ICTRP): data integrity and the trends in clinical trials, diseases, and drugs.Front Pharmacol. 2023 Sep 18;14:1228148. doi: 10.3389/fphar.2023.1228148. eCollection 2023. Front Pharmacol. 2023. PMID: 37790806 Free PMC article.
-
An Update on Glutamatergic System in Suicidal Depression and on the Role of Esketamine.Curr Top Med Chem. 2020;20(7):554-584. doi: 10.2174/1568026620666200131100316. Curr Top Med Chem. 2020. PMID: 32003691
-
Studies registered in non-ClinicalTrials.gov accounted for an increasing proportion of protocol registrations in medical research.J Clin Epidemiol. 2019 Dec;116:106-113. doi: 10.1016/j.jclinepi.2019.09.005. Epub 2019 Sep 12. J Clin Epidemiol. 2019. PMID: 31521723
-
Analysis of Current Status of Clinical Trial Registrations in Andrological Diseases: Insights from ClinicalTrials.gov and ICTRP Databases.Am J Mens Health. 2025 Mar-Apr;19(2):15579883251325478. doi: 10.1177/15579883251325478. Epub 2025 Apr 20. Am J Mens Health. 2025. PMID: 40254740 Free PMC article.
-
Benefits and harms in clinical trials of duloxetine for treatment of major depressive disorder: comparison of clinical study reports, trial registries, and publications.BMJ. 2014 Jun 4;348:g3510. doi: 10.1136/bmj.g3510. BMJ. 2014. PMID: 24899650 Free PMC article. Review.
Cited by
-
Schaftoside Reduces Depression- and Anxiogenic-like Behaviors in Mice Depression Models.Brain Sci. 2025 Feb 24;15(3):238. doi: 10.3390/brainsci15030238. Brain Sci. 2025. PMID: 40149760 Free PMC article.
-
Effectiveness of pharmacological and non-pharmacological interventions for treatment-resistant depression in older patients: a systematic review and meta-analysis.BMJ Ment Health. 2025 Mar 3;28(1):e301324. doi: 10.1136/bmjment-2024-301324. BMJ Ment Health. 2025. PMID: 40032553 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

