A chimeric vaccine derived from Australian genotype IV Japanese encephalitis virus protects mice from lethal challenge
- PMID: 39085247
- PMCID: PMC11291493
- DOI: 10.1038/s41541-024-00903-2
A chimeric vaccine derived from Australian genotype IV Japanese encephalitis virus protects mice from lethal challenge
Abstract
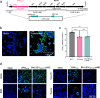
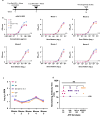
In 2022, a genotype IV (GIV) strain of Japanese encephalitis virus (JEV) caused an unprecedented and widespread outbreak of disease in pigs and humans in Australia. As no veterinary vaccines against JEV are approved in Australia and all current approved human and veterinary vaccines are derived from genotype (G) III JEV strains, we used the recently described insect-specific Binjari virus (BinJV) chimeric flavivirus vaccine technology to produce a JEV GIV vaccine candidate. Herein we describe the production of a chimeric virus displaying the structural prM and E proteins of a JEV GIV isolate obtained from a stillborn piglet (JEVNSW/22) in the genomic backbone of BinJV (BinJ/JEVNSW/22-prME). BinJ/JEVNSW/22-prME was shown to be antigenically indistinguishable from the JEVNSW/22 parental virus by KD analysis and a panel of JEV-reactive monoclonal antibodies in ELISA. BinJ/JEVNSW/22-prME replicated efficiently in C6/36 cells, reaching titres of >107 infectious units/mL - an important attribute for vaccine manufacture. As expected, BinJ/JEVNSW/22-prME failed to replicate in a variety of vertebrate cells lines. When used to immunise mice, the vaccine induced a potent virus neutralising response against JEVNSW/22 and to GII and GIII JEV strains. The BinJ/JEVNSW/22-prME vaccine provided complete protection against lethal challenge with JEVNSW/22, whilst also providing partial protection against viraemia and disease for the related Murray Valley encephalitis virus. Our results demonstrate that BinJ/JEVNSW/22-prME is a promising vaccine candidate against JEV.
© 2024. The Author(s).
Conflict of interest statement
Data included in this publication are based on a patent (WO/2018/176075) on which J.J.H., D.W., H.B-O., R.A.H., and J.H-P. are inventors. The remaining authors declare no competing interests.
Figures



References
-
- Halstead, S., Hills, S. & Dubischar, K. In Plotkin’s Vaccines (eds Plotkin, S. A., Offit, P. A., Orenstien, W. A., & Edwards, K. M.) 511-548e512 (Elsevier, 2018).
-
- Pierson, T. & Diamond, M. In Fields Virology Vol. 1 (eds Knipe, D. M. et al.) Ch. 26 (Lippincott, Williams and Wilkins, 2013).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

