Propofol rescues voltage-dependent gating of HCN1 channel epilepsy mutants
- PMID: 39085604
- PMCID: PMC11634041
- DOI: 10.1038/s41586-024-07743-z
Propofol rescues voltage-dependent gating of HCN1 channel epilepsy mutants
Abstract
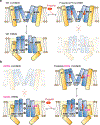
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels1 are essential for pacemaking activity and neural signalling2,3. Drugs inhibiting HCN1 are promising candidates for management of neuropathic pain4 and epileptic seizures5. The general anaesthetic propofol (2,6-di-iso-propylphenol) is a known HCN1 allosteric inhibitor6 with unknown structural basis. Here, using single-particle cryo-electron microscopy and electrophysiology, we show that propofol inhibits HCN1 by binding to a mechanistic hotspot in a groove between the S5 and S6 transmembrane helices. We found that propofol restored voltage-dependent closing in two HCN1 epilepsy-associated polymorphisms that act by destabilizing the channel closed state: M305L, located in the propofol-binding site in S5, and D401H in S6 (refs. 7,8). To understand the mechanism of propofol inhibition and restoration of voltage-gating, we tracked voltage-sensor movement in spHCN channels and found that propofol inhibition is independent of voltage-sensor conformational changes. Mutations at the homologous methionine in spHCN and an adjacent conserved phenylalanine in S6 similarly destabilize closing without disrupting voltage-sensor movements, indicating that voltage-dependent closure requires this interface intact. We propose a model for voltage-dependent gating in which propofol stabilizes coupling between the voltage sensor and pore at this conserved methionine-phenylalanine interface in HCN channels. These findings unlock potential exploitation of this site to design specific drugs targeting HCN channelopathies.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest declaration
GT and PG are co-inventors on patents related to the development of novel alkylphenols for the treatment of neuropathic pain. GT and PG serve on the Scientific Advisory Board for Akelos Inc. (New York, NY), a research-based biotechnology company that has a licensing agreement for the use of those patents.
Figures


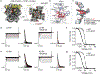










Comment in
-
From General Anesthetic to Designer Antiepileptic: Propofol's Mechanism of Action Reveals Hope for Precision Treatment of HCN1-Related Epilepsy.Epilepsy Curr. 2025 Feb 28;25(3):192-194. doi: 10.1177/15357597251318573. eCollection 2025 May-Jun. Epilepsy Curr. 2025. PMID: 40028189 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

