The DNA repair protein DNA-PKcs modulates synaptic plasticity via PSD-95 phosphorylation and stability
- PMID: 39085642
- PMCID: PMC11315936
- DOI: 10.1038/s44319-024-00198-3
The DNA repair protein DNA-PKcs modulates synaptic plasticity via PSD-95 phosphorylation and stability
Abstract
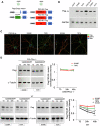
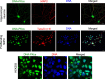
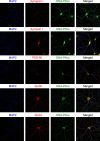
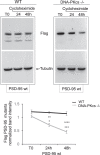
The key DNA repair enzyme DNA-PKcs has several and important cellular functions. Loss of DNA-PKcs activity in mice has revealed essential roles in immune and nervous systems. In humans, DNA-PKcs is a critical factor for brain development and function since mutation of the prkdc gene causes severe neurological deficits such as microcephaly and seizures, predicting yet unknown roles of DNA-PKcs in neurons. Here we show that DNA-PKcs modulates synaptic plasticity. We demonstrate that DNA-PKcs localizes at synapses and phosphorylates PSD-95 at newly identified residues controlling PSD-95 protein stability. DNA-PKcs -/- mice are characterized by impaired Long-Term Potentiation (LTP), changes in neuronal morphology, and reduced levels of postsynaptic proteins. A PSD-95 mutant that is constitutively phosphorylated rescues LTP impairment when over-expressed in DNA-PKcs -/- mice. Our study identifies an emergent physiological function of DNA-PKcs in regulating neuronal plasticity, beyond genome stability.
Keywords: Cognitive Function; DNA Repair; DNA-PKcs; PSD-95 Phosphorylation; Synaptic Plasticity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Anderson CW, Lees-Miller SP (1992) The nuclear serine/threonine protein kinase DNA-PK. Crit Rev Eukaryot Gene Expr 2:283–314 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

