Prevention and treatment of peri-implant fibrosis by functionally inhibiting skeletal cells expressing the leptin receptor
- PMID: 39085645
- PMCID: PMC12016487
- DOI: 10.1038/s41551-024-01238-y
Prevention and treatment of peri-implant fibrosis by functionally inhibiting skeletal cells expressing the leptin receptor
Abstract
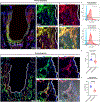
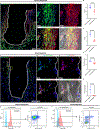
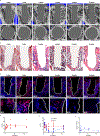
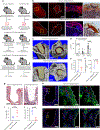
The cellular and molecular mediators of peri-implant fibrosis-a most common reason for implant failure and for surgical revision after the replacement of a prosthetic joint-remain unclear. Here we show that peri-implant fibrotic tissue in mice and humans is largely composed of a specific population of skeletal cells expressing the leptin receptor (LEPR) and that these cells are necessary and sufficient to generate and maintain peri-implant fibrotic tissue. In a mouse model of tibial implantation and osseointegration that mimics partial knee arthroplasty, genetic ablation of LEPR+ cells prevented peri-implant fibrosis and the implantation of LEPR+ cells from peri-implant fibrotic tissue was sufficient to induce fibrosis in secondary hosts. Conditional deletion of the adhesion G-protein-coupled receptor F5 (ADGRF5) in LEPR+ cells attenuated peri-implant fibrosis while augmenting peri-implant bone formation, and ADGRF5 inhibition by the intra-articular or systemic administration of neutralizing anti-ADGRF5 in the mice prevented and reversed peri-implant fibrosis. Pharmaceutical agents that inhibit the ADGRF5 pathway in LEPR+ cells may be used to prevent and treat peri-implant fibrosis.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures














References
-
- Shah PK Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation 107, 2175–2177 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

