A Cloud-Based System for Automated AI Image Analysis and Reporting
- PMID: 39085717
- PMCID: PMC11811354
- DOI: 10.1007/s10278-024-01200-z
A Cloud-Based System for Automated AI Image Analysis and Reporting
Abstract
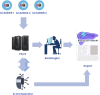
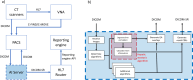
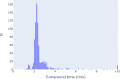
Although numerous AI algorithms have been published, the relatively small number of algorithms used clinically is partly due to the difficulty of implementing AI seamlessly into the clinical workflow for radiologists and for their healthcare enterprise. The authors developed an AI orchestrator to facilitate the deployment and use of AI tools in a large multi-site university healthcare system and used it to conduct opportunistic screening for hepatic steatosis. During the 60-day study period, 991 abdominal CTs were processed at multiple different physical locations with an average turnaround time of 2.8 min. Quality control images and AI results were fully integrated into the existing clinical workflow. All input into and output from the server was in standardized data formats. The authors describe the methodology in detail; this framework can be adapted to integrate any clinical AI algorithm.
Keywords: AI; AI orchestrator; Informatics; Opportunistic screening; Steatosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: The project was reviewed by the Institutional Review Board of the University of Pennsylvania on March 22, 2021 (IRB protocol #848519). A waiver of informed consent was requested and granted due to minimal risk, retrospective nature of the study, and deidentification of patient identifying information. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Consent to Participate: As detailed in the “Ethics Approval” section, this study was reviewed by our IRB and performed under a waiver for informed consent. Consent to Publish: As detailed in the “Ethics Approval” section, this study was reviewed by our IRB and performed under a waiver for informed consent. Competing Interest: The authors declare no competing interests.
Figures



References
-
- Artificial intelligence and machine learning (AI/ML)-enabled medical devices. https://www.fda.gov/medical-devices/software-medical-device-samd/artific.... - PMC - PubMed
-
- Bates DDB, Pickhardt PJ. CT-derived body composition assessment as a prognostic tool in oncologic patients: From opportunistic research to artificial intelligence–based clinical implementation. American Journal of Roentgenology. 2022;219(4):671–680. 10.2214/AJR.22.27749. - PubMed
-
- Buls N, Watté N, Nieboer K, Ilsen B, de Mey J. Performance of an artificial intelligence tool with real-time clinical workflow integration – Detection of intracranial hemorrhage and pulmonary embolism. Physica Medica. 2021;83:154–160. 10.1016/j.ejmp.2021.03.015. - PubMed
-
- Shoshan Y, Bakalo R, Gilboa-Solomon F, et al. Artificial intelligence for reducing workload in breast cancer screening with digital breast tomosynthesis. Radiology. 2022;303(1):69–77. 10.1148/radiol.211105. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
