Static magnetic field-modulated mesenchymal stem cell-derived mitochondria-containing microvesicles for enhanced intervertebral disc degeneration therapy
- PMID: 39085827
- PMCID: PMC11290117
- DOI: 10.1186/s12951-024-02728-6
Static magnetic field-modulated mesenchymal stem cell-derived mitochondria-containing microvesicles for enhanced intervertebral disc degeneration therapy
Abstract
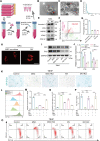
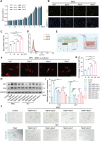
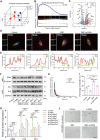
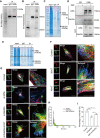
Intervertebral disc degeneration (IVDD) is characterized by the senescence and declining vitality of nucleus pulposus cells (NPCs), often driven by mitochondrial dysfunction. This study elucidates that mesenchymal stem cells (MSCs) play a crucial role in attenuating NPC senescence by secreting mitochondria-containing microvesicles (mitoMVs). Moreover, it demonstrates that static magnetic fields (SMF) enhance the secretion of mitoMVs by MSCs. By distinguishing mitoMV generation from exosomes, this study shifts focus to understanding the molecular mechanisms of SMF intervention, emphasizing cargo transport and plasma membrane budding processes, with RNA sequencing indicating the potential involvement of the microtubule-based transport protein Kif5b. The study further confirms the interaction between Rab22a and Kif5b, revealing Rab22a's role in sorting mitoMVs into microvesicles (MVs) and potentially mediating subsequent plasma membrane budding. Subsequent construction of a gelatin methacrylate (GelMA) hydrogel delivery system further addresses the challenges of in vivo application and verifies the substantial potential of mitoMVs in delaying IVDD. This research not only sheds light on the molecular intricacies of SMF-enhanced mitoMV secretion but also provides innovative perspectives for future IVDD therapeutic strategies.
Keywords: Intervertebral disc degeneration; Mesenchymal stem cell; Microvesicle; Mitochondrial; Static magnetic field.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

