Development and IND-enabling studies of a novel Cas9 genome-edited autologous CD34+ cell therapy to induce fetal hemoglobin for sickle cell disease
- PMID: 39086133
- PMCID: PMC11489559
- DOI: 10.1016/j.ymthe.2024.07.022
Development and IND-enabling studies of a novel Cas9 genome-edited autologous CD34+ cell therapy to induce fetal hemoglobin for sickle cell disease
Abstract
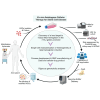
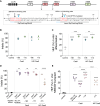
Sickle cell disease (SCD) is a common, severe genetic blood disorder. Current pharmacotherapies are partially effective and allogeneic hematopoietic stem cell transplantation is associated with immune toxicities. Genome editing of patient hematopoietic stem cells (HSCs) to reactivate fetal hemoglobin (HbF) in erythroid progeny offers an alternative potentially curative approach to treat SCD. Although the FDA released guidelines for evaluating genome editing risks, it remains unclear how best to approach pre-clinical assessment of genome-edited cell products. Here, we describe rigorous pre-clinical development of a therapeutic γ-globin gene promoter editing strategy that supported an investigational new drug application cleared by the FDA. We compared γ-globin promoter and BCL11A enhancer targets, identified a potent HbF-inducing lead candidate, and tested our approach in mobilized CD34+ hematopoietic stem progenitor cells (HSPCs) from SCD patients. We observed efficient editing, HbF induction to predicted therapeutic levels, and reduced sickling. With single-cell analyses, we defined the heterogeneity of HbF induction and HBG1/HBG2 transcription. With CHANGE-seq for sensitive and unbiased off-target discovery followed by targeted sequencing, we did not detect off-target activity in edited HSPCs. Our study provides a blueprint for translating new ex vivo HSC genome editing strategies toward clinical trials for treating SCD and other blood disorders.
Keywords: CRISPR-Cas9; HSCs; autologous cellular therapy; ex vivo; fetal hemoglobin; gene therapy; genome editing; sickle cell disease; γ-gamma globin promoter editing.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.S. has received consultant fees from Spotlight Therapeutics, Medexus Inc., Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine. He is a medical monitor for RCI BMT CSIDE clinical trials, for which he receives financial compensation. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. A.S. is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. has no direct financial interest in these therapies. J.S.Y. is an equity owner of Beam Therapeutics. M.J.W. is on advisory boards for Cellarity Inc., Novartis, and Forma Therapeutics. S.Q.T. is a co-inventor on licensed patents for CHANGE-seq and other genome engineering technologies. S.Q.T. is a member of the scientific advisory boards of Prime Medicine and Ensoma.
Figures




References
-
- Piel F.B., Steinberg M.H., Rees D.C. Sickle cell disease. N. Engl. J. Med. 2017;377:305. - PubMed
-
- Ingram V.M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178:792–794. - PubMed
-
- Bunn H.F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337:762–769. - PubMed
-
- Kato G.J., Piel F.B., Reid C.D., Gaston M.H., Ohene-Frempong K., Krishnamurti L., Smith W.R., Panepinto J.A., Weatherall D.J., Costa F.F., Vichinsky E.P. Sickle cell disease. Nat. Rev. Dis. Primers. 2018;4 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

