Zonation and ligand and dose dependence of sphingosine 1-phosphate receptor-1 signalling in blood and lymphatic vasculature
- PMID: 39086170
- PMCID: PMC11587562
- DOI: 10.1093/cvr/cvae168
Zonation and ligand and dose dependence of sphingosine 1-phosphate receptor-1 signalling in blood and lymphatic vasculature
Abstract
Aims: Circulating levels of sphingosine 1-phosphate (S1P), an HDL-associated ligand for the endothelial cell (EC) protective S1P receptor-1 (S1PR1), are reduced in disease states associated with endothelial dysfunction. Yet, as S1PR1 has high affinity for S1P and can be activated by ligand-independent mechanisms and EC autonomous S1P production, it is unclear if relative reductions in circulating S1P can cause endothelial dysfunction. It is also unclear how EC S1PR1 insufficiency, whether induced by deficiency in circulating ligand or by S1PR1-directed immunosuppressive therapy, affects different vascular subsets.
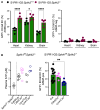
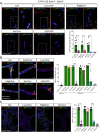
Methods and results: We here fine map the zonation of S1PR1 signalling in the murine blood and lymphatic vasculature, superimpose cell-type-specific and relative deficiencies in S1P production to define ligand source and dose dependence, and correlate receptor engagement to essential functions. In naïve blood vessels, despite broad expression, EC S1PR1 engagement was restricted to resistance-size arteries, lung capillaries, and a subset of high-endothelial venules (HEVs). Similar zonation was observed for albumin extravasation in EC S1PR1-deficient mice, and brain extravasation was reproduced with arterial EC-selective S1pr1 deletion. In lymphatic ECs, S1PR1 engagement was high in collecting vessels and lymph nodes and low in blind-ended capillaries that drain tissue fluids. While EC S1P production sustained S1PR1 signalling in lymphatics and HEV, haematopoietic cells provided ∼90% of plasma S1P and sustained signalling in resistance arteries and lung capillaries. S1PR1 signalling and endothelial function were both surprisingly sensitive to reductions in plasma S1P with apparent saturation around 50% of normal levels. S1PR1 engagement did not depend on sex or age but modestly increased in arteries in hypertension and diabetes. Sphingosine kinase (Sphk)-2 deficiency also increased S1PR1 engagement selectively in arteries, which could be attributed to Sphk1-dependent S1P release from perivascular macrophages.
Conclusion: This study highlights vessel subtype-specific S1PR1 functions and mechanisms of engagement and supports the relevance of S1P as circulating biomarker for endothelial function.
Keywords: Endothelial function; Signalling; Sphingosine 1-phosphate; Vascular biology; Vascular integrity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures

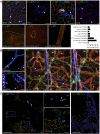
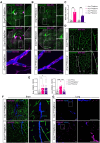
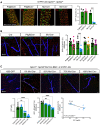
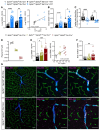

Comment in
-
Uncovering vascular signature of sphingosine-1-phosphate receptor 1 activation.Cardiovasc Res. 2024 Nov 25;120(14):1657-1659. doi: 10.1093/cvr/cvae206. Cardiovasc Res. 2024. PMID: 39259287 No abstract available.
References
-
- Baeyens AAL, Schwab SR. Finding a way out: S1P signaling and immune cell migration. Annu Rev Immunol 2020;38:759–784. - PubMed
-
- Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003;102:3665–3667. - PubMed
-
- Gaengel K, Niaudet C, Hagikura K, Laviña B, Muhl L, Hofmann JJ, Ebarasi L, Nyström S, Rymo S, Chen LL, Pang M-F, Jin Y, Raschperger E, Roswall P, Schulte D, Benedito R, Larsson J, Hellström M, Fuxe J, Uhlén P, Adams R, Jakobsson L, Majumdar A, Vestweber D, Uv A, Betsholtz C. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 2012;23:587–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

