Primitive macrophages induce sarcomeric maturation and functional enhancement of developing human cardiac microtissues via efferocytic pathways
- PMID: 39086373
- PMCID: PMC11290557
- DOI: 10.1038/s44161-024-00471-7
Primitive macrophages induce sarcomeric maturation and functional enhancement of developing human cardiac microtissues via efferocytic pathways
Abstract
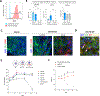
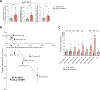
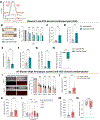
Yolk sac macrophages are the first to seed the developing heart, however we have no understanding of their roles in human heart development and function due to a lack of accessible tissue. Here, we bridge this gap by differentiating human embryonic stem cells (hESCs) into primitive LYVE1+ macrophages (hESC-macrophages) that stably engraft within contractile cardiac microtissues composed of hESC-cardiomyocytes and fibroblasts. Engraftment induces a human fetal cardiac macrophage gene program enriched in efferocytic pathways. Functionally, hESC-macrophages trigger cardiomyocyte sarcomeric protein maturation, enhance contractile force and improve relaxation kinetics. Mechanistically, hESC-macrophages engage in phosphatidylserine dependent ingestion of apoptotic cardiomyocyte cargo, which reduces microtissue stress, leading hESC-cardiomyocytes to more closely resemble early human fetal ventricular cardiomyocytes, both transcriptionally and metabolically. Inhibiting hESC-macrophage efferocytosis impairs sarcomeric protein maturation and reduces cardiac microtissue function. Taken together, macrophage-engineered human cardiac microtissues represent a considerably improved model for human heart development, and reveal a major beneficial role for human primitive macrophages in enhancing early cardiac tissue function.
Conflict of interest statement
Competing interests M. R. and Y.Z. are inventors on patents for cardiac tissue cultivation that are licensed to Valo Health. They receive licensing royalty from this invention. Q.W., M.R., and Y.Z. have a filed patent application on thermoplastic polymer composition for micro 3D printing and uses thereof. All other authors declare no competing interests.
Figures





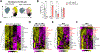









References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
