Anti-IL23/12 agents and JAK inhibitors for inflammatory bowel disease
- PMID: 39086483
- PMCID: PMC11288814
- DOI: 10.3389/fimmu.2024.1393463
Anti-IL23/12 agents and JAK inhibitors for inflammatory bowel disease
Abstract
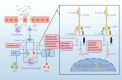
IBD (inflammatory bowel disease) is a chronic inflammatory disease of the gastrointestinal tract with increasing incidence worldwide. Multiple factors, such as genetic background, environmental and luminal factors, and mucosal immune dysregulation, have been implicated in the cause of IBD, although the cause of the disease remains unknown. IL-12 and IL-23 and their downstream signaling pathways participate in the pathogenesis of inflammatory bowel disease. Early and aggressive treatment with biologic therapies or novel small molecules is needed to decrease complications and the need for hospitalization and surgery. The landscape of inflammatory bowel disease (IBD) treatment has tremendously improved with the development of biologics and small molecule drugs. Several novel biologics and small molecule drugs targeting IL-12 and IL-23 and their downstream targets have shown positive efficacy and safety data in clinical trials, and several drugs have been approved for the treatment of IBD. In the future, numerous potential emerging therapeutic options for IBD treatment are believed to come to the fore, achieving disease cure.
Keywords: IL-12/23 p40 subunit; IL-23 p19 subunit; JAK inhibitors; JAK/STAT signaling; anti-IL12/23 biologics; inflammatory bowel disease; proteolysis targeting chimera (PROTAC).
Copyright © 2024 Tian, Zhao and Teng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

