Vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication in Chinese population: A prospective, multicenter, randomized, two-stage study
- PMID: 39086752
- PMCID: PMC11287422
- DOI: 10.3748/wjg.v30.i27.3304
Vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication in Chinese population: A prospective, multicenter, randomized, two-stage study
Abstract
Background: The efficacy of Vonoprazan-amoxicillin dual therapy (VAT) in the treatment of Helicobacter pylori (H. pylori) is controversial.
Aim: To evaluate the efficacy of VAT in the Chinese population.
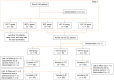
Methods: This prospective, multicenter, randomized, open-label, and two-stage study was conducted at 23 centers in Fujian, China (May 2021-April 2022). H. pylori-infected patients were randomized to bismuth quadruple therapy (BQT), BQT-Vonoprazan (BQT-V), seven-day VAT (VAT-7), ten-day VAT (VAT-10), and fourteen-day VAT (VAT-14) groups. The primary endpoint was the H. pylori eradication rate. The secondary endpoint was the frequency of adverse events. This study was registered with the Chinese Clinical Trial Registry, ChiCTR2100045778.
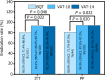
Results: In the first stage, VAT-7 and BQT-V groups were selected for early termination because less than 23 among 28 cases were eradicated. In the second stage, the eradication rates for BQT, VAT-10, and VA-14 were 80.2% [95% confidence interval (95%CI): 71.4%-86.8%], 93.2% (86.6%-96.7%), 92.2% (85.3%-96.0%) in the intention-to-treat (ITT) analysis, and 80.9% (95%CI: 71.7%-87.5%), 94.0% (87.5%-97.2%), and 93.9% (87.4%-97.2%) in the per-protocol analysis. The ITT analysis showed a higher eradication rate in the VAT-10 and VAT-14 groups than in the BQT group (P = 0.022 and P = 0.046, respectively). The incidence of adverse events in the VAT-10 and VAT-14 groups was lower than in the BQT group (25.27% and 13.73% vs 37.62%, respectively; P < 0.001).
Conclusion: VAT with a duration of 10 or 14 days achieves a higher eradication rate than the BQT, with a more tolerable safety profile in H. pylori-infected patients in Fujian.
Keywords: Amoxicillin; Bismuth quadruple therapy; Dual therapy; Helicobacter pylori; Vonoprazan.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest to disclose.
Figures
References
-
- Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22:Suppl 1. - PubMed
-
- Megraud F, Bruyndonckx R, Coenen S, Wittkop L, Huang TD, Hoebeke M, Bénéjat L, Lehours P, Goossens H, Glupczynski Y European Helicobacter pylori Antimicrobial Susceptibility Testing Working Group. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70:1815–1822. - PubMed
-
- Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. - PubMed
-
- Unge P, Gad A, Gnarpe H, Olsson J. Does omeprazole improve antimicrobial therapy directed towards gastric Campylobacter pylori in patients with antral gastritis? A pilot study. Scand J Gastroenterol Suppl. 1989;167:49–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

