Switching within versus out of class following first-line TNFi failure in ulcerative colitis: real-world outcomes from a German claims data analysis
- PMID: 39086989
- PMCID: PMC11289825
- DOI: 10.1177/17562848241262288
Switching within versus out of class following first-line TNFi failure in ulcerative colitis: real-world outcomes from a German claims data analysis
Abstract
Background: Biologic agents have demonstrated efficacy in treating ulcerative colitis (UC); however, treatment failure to tumor necrosis factor inhibitors (TNFi) is common in the real world. Data on preferential sequencing in clinical practice after failure remain limited.
Objectives: This study aimed to evaluate real-world outcomes of patients cycling to TNFis or switching to non-TNFi biologics following first-line failure with TNFis.
Design: Retrospective cohort study in Germany.
Methods: Adult patients with UC were identified using administrative claims data from 1 May 2014 to 30 June 2022 provided by a statutory sickness fund. Patients newly initiating first-line therapy with TNFis and then switching to another agent were identified. Patients were defined as within-class switched (WCS), if they cycled to another TNFi, or outside-class switchers (OCS), if they switched to a non-TNFi biologic [ustekinumab (UST) or vedolizumab (VDZ)] and followed from index (switch date) to death, insurance end, or study end on 30 June 2022. Inverse probability of treatment weighting (IPTW) was performed to adjust for differences in baseline characteristics between groups, and weighted Cox regression models were used to compare primary (time to discontinuation and second treatment switch) and secondary outcomes (corticosteroid-free drug survival).
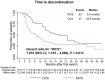
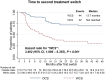
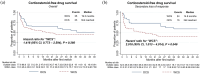
Results: We identified 166 patients initiating TNFis and switching to a subsequent treatment (mean age: 42.9 years, 49.4% female). Following IPTW, there were 71 and 76 patients in the WCS and OCS groups, respectively. Compared to OCS, WCS were more likely to discontinue the new therapy [hazard ratio (HR), 1.82, 95% confidence interval (CI), 1.14-2.89, p = 0.012], and switch a second time (HR, 3.46, 95% CI, 1.89-6.36, p < 0.001). Moreover, WCS showed an increased likelihood of initiating prolonged corticosteroid therapy (HR, 1.42, 95% CI, 0.77-2.59, p = 0.260); however, the results were not significant.
Conclusion: Following first-line TNFi failure, this study suggests that real-world outcomes among patients with UC are less favorable when cycling to another TNFi, compared to switching to a non-TNFi such as UST or VDZ.
Keywords: TNF-cycling; biologics; claims data; switching; therapy sequence; ulcerative colitis.
© The Author(s), 2024.
Conflict of interest statement
DW, RN, IB, DZ, AD, and JL are employees of Janssen-Cilag. EZ is an employee of Cytel Inc., whose work was funded by Janssen-Cilag. AF participated in this study as part of AOK PLUS. SM and TW are staff members of IPAM. TW has received honoraria from several pharmaceutical/consultancy firms, for example, NovoNordisk, Abbvie, Merck, GSK, BMS, LEO Pharma, Bayer, and Boehringer Ingelheim. BB has received consultancy fees and grants from several pharmaceutical companies, for example, AbbVie, MSD, Ferring, Falk, Takeda, Shield Therapeutics, Pfizer, Biogen, BMS, Janssen, Celltrion, and Galapagos.
Figures


Similar articles
-
Real-world outcomes associated with switching to anti-TNFs versus other biologics in Crohn's Disease patients: A retrospective analysis using German claims data.Therap Adv Gastroenterol. 2022 Nov 4;15:17562848221130554. doi: 10.1177/17562848221130554. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36353736 Free PMC article.
-
Treatment Persistence Among Anti-Tumor Necrosis Factor-experienced Patients With Ulcerative Colitis Switching to a Biologic With a Different Mode of Action or Cycling to Another Anti-Tumor Necrosis Factor Agent.Clin Ther. 2025 Mar;47(3):204-211. doi: 10.1016/j.clinthera.2024.12.002. Epub 2024 Dec 31. Clin Ther. 2025. PMID: 39743427
-
Persistence Among Patients with Crohn Disease Previously Treated with an Anti-tumor Necrosis Factor Inhibitor and Switching or Cycling to Another Biologic Agent.Clin Ther. 2023 Aug;45(8):770-777. doi: 10.1016/j.clinthera.2023.06.013. Epub 2023 Jul 11. Clin Ther. 2023. PMID: 37442653
-
Real-World Patient Experience of Switching Biologic Treatment in Inflammatory Arthritis and Ulcerative Colitis - A Systematic Literature Review.Patient Prefer Adherence. 2020 Feb 17;14:309-320. doi: 10.2147/PPA.S238843. eCollection 2020. Patient Prefer Adherence. 2020. PMID: 32109997 Free PMC article. Review.
-
Evaluation of the risk of heart failure with tumour necrosis factor inhibitors: A large-scale meta-analysis in immune-mediated inflammatory diseases.J Eur Acad Dermatol Venereol. 2025 Jun 11. doi: 10.1111/jdv.20786. Online ahead of print. J Eur Acad Dermatol Venereol. 2025. PMID: 40497591 Review.
Cited by
-
Effectiveness and Safety of a Second JAK Inhibitor in Ulcerative Colitis: The J2J Multicentre Study.Aliment Pharmacol Ther. 2025 Aug;62(4):430-439. doi: 10.1111/apt.70199. Epub 2025 May 22. Aliment Pharmacol Ther. 2025. PMID: 40400411 Free PMC article.
References
-
- Dignass A, Preiss JC, Aust DE, et al.. [Updated German guideline on diagnosis and treatment of ulcerative colitis, 2011]. Z Gastroenterol 2011; 49: 1276–1341. - PubMed
-
- Geiss T, Schaefert RM, Berens S, et al.. Risk of depression in patients with inflammatory bowel disease. J Dig Dis 2018; 19: 456–467. - PubMed
-
- Raine T, Bonovas S, Burisch J, et al.. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022; 16: 2–17. - PubMed
LinkOut - more resources
Full Text Sources

