DOMINO trial post hoc analysis: evaluation of the diet effects on symptoms in IBS subtypes
- PMID: 39086991
- PMCID: PMC11289810
- DOI: 10.1177/17562848241255296
DOMINO trial post hoc analysis: evaluation of the diet effects on symptoms in IBS subtypes
Abstract
Background: Irritable bowel syndrome (IBS) is a disorder of gut-brain interaction characterized by recurrent abdominal pain related to defecation and/or associated to a change in bowel habits. According to the stool type, four different IBS subtypes can be recognized, constipation predominant (IBS-C), diarrhea predominant (IBS-D), mixed (IBS-M), and undefined (IBS-U). Patients report that their IBS symptoms are exacerbated by food. Thus, it is important to find a nutritional approach that could be effective in reducing IBS symptoms.
Objective: The present work is a post hoc analysis of the previously published DOMINO trial. It aimed to evaluate the effects of a self-instructed FODMAP-lowering diet smartphone application on symptoms and psychosocial aspects in primary care IBS stratifying the results for each IBS subtypes.
Design: Post hoc analysis.
Methods: Two hundred twenty-two primary care IBS patients followed a FODMAP-lowering diet for 8 weeks with the support of a smartphone application. Two follow-up visits were scheduled after 16 and 24 weeks. IBS-Symptoms Severity Score (IBS-SSS), quality of life (QoL), and adherence and dietary satisfaction were evaluated.
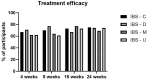
Results: After 8 weeks, IBS-SSS improved in all IBS subtypes (p < 0.0001). Physician Health Questiionnaire (PHQ-15) improved only in IBS-D (p = 0.0006), whereas QoL improved both in IBS-D (p = 0.01) and IBS-M (p = 0.005).
Conclusion: This post hoc analysis showed that the app is useful in all IBS subtypes; thus, it could be used as an effective tool by both general practitioners and patients to manage symptoms in primary care.
Trial registration: Ethical Commission University Hospital of Leuven reference number: S59482. Clinicaltrial.gov reference number: NCT04270487.
Keywords: FODMAP diet; IBS subtypes; dietary treatment in IBS.
Plain language summary
What is already known about this subject? The low FODMAP (fermentable oligo-, di-, and monosaccharides and polyols) diet has shown efficacy for controlling IBS (irritable bowel syndrome) symptoms in small controlled trials in tertiary care patients. As this approach requires several visits with an experienced dietitian, it seems less suitable for primary care. What are the new findings? The benefit of the FODMAP lowering app was already present at 4 weeks and persisted during follow-up until 24 weeks. How might it impact on clinical practice in the foreseeable future? Given its superiority to standard first-line pharmacotherapy, and its ease of use, a FODMAP lowering app has the potential to become the preferred first-line treatment for primary care IBS.
© The Author(s), 2024.
Conflict of interest statement
Jan Tack has given Scientific advice to AlfaWassermann, Allergan, Christian Hansen, Danone, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neutec, Novartis, Noventure, Nutricia, Shionogi, Shire, Takeda, Theravance, Tramedico, Truvion, Tsumura, Zealand and Zeria pharmaceuticals, has received research support from Shire, Sofar and Tsumura, and has served on the Speaker bureau for Abbott, Allergan, AstraZeneca, Janssen, Kyowa Kirin, Menarini and Karen PDM. Christophe Matthys has served on the Speaker bureau for Coca-Cola and Zespri and received travel/conference grants from Danone, Nestlé Health Sciences, Fresenius-Kabi. This study was supported by a research grant from the Belgian Health Care Knowledge Centre (KCE). Questionnaires in this trial were developed, translated, and provided by the Rome Foundation Research Institute.
Figures



References
-
- Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021; 160: 99–114. - PubMed
-
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407.e1395. - PubMed
-
- Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 2020; 116: 17–44. - PubMed
-
- Seamark L, Barclay Y, Marchant C, et al. Long-term symptom severity in people with irritable bowel syndrome following dietetic treatment in primary care: a service evaluation. J Hum Nutr Diet 2021; 34: 890–900. - PubMed
-
- Kindt S, Louis H, De Schepper H, et al. Belgian consensus on irritable bowel syndrome. Acta Gastroenterol Belg 2022; 85: 360–382. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

