Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer's disease-related amyloid pathology
- PMID: 39087029
- PMCID: PMC7616318
- DOI: 10.1038/s44161-024-00445-9
Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer's disease-related amyloid pathology
Abstract
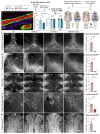
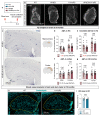
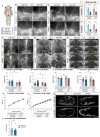
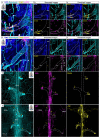
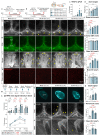
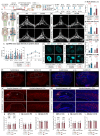
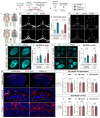
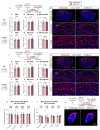
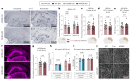
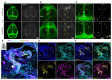
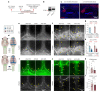
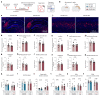
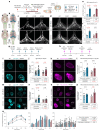
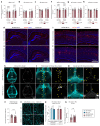
Discovery of meningeal lymphatic vessels (LVs) in the dura mater, also known as dural LVs (dLVs) that depend on vascular endothelial growth factor C expression, has raised interest in their possible involvement in Alzheimer's disease (AD). Here we find that in the APdE9 and 5xFAD mouse models of AD, dural amyloid-β (Aβ) is confined to blood vessels and dLV morphology or function is not altered. The induction of sustained dLV atrophy or hyperplasia in the AD mice by blocking or overexpressing vascular endothelial growth factor C, impaired or improved, respectively, macromolecular cerebrospinal fluid (CSF) drainage to cervical lymph nodes. Yet, sustained manipulation of dLVs did not significantly alter the overall brain Aβ plaque load. Moreover, dLV atrophy did not alter the behavioral phenotypes of the AD mice, but it improved CSF-to-blood drainage. Our results indicate that sustained dLV manipulation does not affect Aβ deposition in the brain and that compensatory mechanisms promote CSF clearance.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
