Enhancing the Intrinsic Antiplasmodial Activity and Improving the Stability and Selectivity of a Tunable Peptide Scaffold Derived from Human Platelet Factor 4
- PMID: 39087267
- PMCID: PMC11320574
- DOI: 10.1021/acsinfecdis.4c00276
Enhancing the Intrinsic Antiplasmodial Activity and Improving the Stability and Selectivity of a Tunable Peptide Scaffold Derived from Human Platelet Factor 4
Abstract
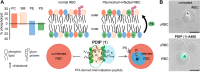
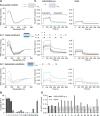
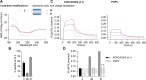
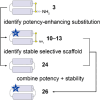
The control of malaria, a disease caused by Plasmodium parasites that kills over half a million people every year, is threatened by the continual emergence and spread of drug resistance. Therefore, new molecules with different mechanisms of action are needed in the antimalarial drug development pipeline. Peptides developed from host defense molecules are gaining traction as anti-infectives due to theood of inducing drug resistance. Human platelet factor 4 (PF4) has intrinsic activity against P. falciparum, and a macrocyclic helix-loop-helix peptide derived from its active domain recapitulates this activity. In this study, we used a stepwise approach to optimize first-generation PF4-derived internalization peptides (PDIPs) by producing analogues with substitutions to charged and hydrophobic amino acid residues or with modifications to terminal residues including backbone cyclization. We evaluated the in vitro activity of PDIP analogues against P. falciparum compared to their overall helical structure, resistance to breakdown by serum proteases, selective binding to negatively charged membranes, and hemolytic activity. Next, we combined antiplasmodial potency-enhancing substitutions that retained favorable membrane and cell-selective properties onto the most stable scaffold to produce a backbone cyclic PDIP analogue with four-fold improved activity against P. falciparum compared to first-generation peptides. These studies demonstrate the ability to modify PDIP to select for and combine desirable properties and further validate the suitability of this unique peptide scaffold for developing a new molecule class that is distinct from existing antimalarial drugs.
Keywords: Plasmodium; drug development; host defense peptide; malaria; rational design; targeted cell-penetration.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- World Health Organisation . World Malaria Report 2023; Licence: CC BY-NC-SA 3.0 IGO: Geneva, 2023.
-
- Balikagala B.; Fukuda N.; Ikeda M.; Katuro O. T.; Tachibana S.-I.; Yamauchi M.; Opio W.; Emoto S.; Anywar D. A.; Kimura E.; Palacpac N. M. Q.; Odongo-Aginya E. I.; Ogwang M.; Horii T.; Mita T. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 2021, 385 (13), 1163–1171. 10.1056/NEJMoa2101746. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

