Novel Inhibition of Central Carbon Metabolism Pathways by Rac and CDC42 inhibitor MBQ167 and Paclitaxel
- PMID: 39087451
- PMCID: PMC11534544
- DOI: 10.1158/1535-7163.MCT-23-0803
Novel Inhibition of Central Carbon Metabolism Pathways by Rac and CDC42 inhibitor MBQ167 and Paclitaxel
Abstract
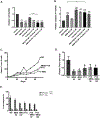
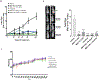
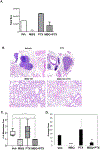
Triple negative breast cancer (TNBC) represents a therapeutic challenge in which standard chemotherapy is limited to paclitaxel. MBQ167, a clinical stage small molecule inhibitor that targets Rac and Cdc42, inhibits tumor growth and metastasis in mouse models of TNBC. Herein, we investigated the efficacy of MBQ167 in combination with paclitaxel in TNBC preclinical models, as a prelude to safety trials of this combination in patients with advanced breast cancer. Individual MBQ167 or combination therapy with paclitaxel was more effective at reducing TNBC cell viability and increasing apoptosis compared with paclitaxel alone. In orthotopic mouse models of human TNBC (MDA-MB231 and MDA-MB468), individual MBQ167, paclitaxel, or the combination reduced mammary tumor growth with similar efficacy, with no apparent liver toxicity. However, paclitaxel single agent treatment significantly increased lung metastasis, whereas MBQ167, single or combined, reduced lung metastasis. In the syngeneic 4T1/BALB/c model, combined MBQ167 and paclitaxel decreased established lung metastases by ∼80%. To determine the molecular basis for the improved efficacy of the combined treatment on metastasis, 4T1 tumor extracts from BALB/c mice treated with MBQ167, paclitaxel, or the combination were subjected to transcriptomic analysis. Gene set enrichment identified specific downregulation of central carbon metabolic pathways by the combination of MBQ167 and paclitaxel but not individual compounds. Biochemical validation, by immunoblotting and metabolic Seahorse analysis, shows that combined MBQ167 and paclitaxel reduces glycolysis. This study provides a strong rationale for the clinical testing of MBQ167 in combination with paclitaxel as a potential therapeutic for TNBC and identifies a unique mechanism of action.
©2024 American Association for Cancer Research.
Conflict of interest statement
Authors' Disclosures
SD owns stock at MBQ Pharma, Inc., which has licensed patents US 9,981,980, US10,392,396 and international patents related to PCT/US2017/029921 relevant to this work from the University of Puerto Rico. The other authors declare no conflicts of interest.
Figures


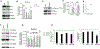
References
-
- Lehmann BD, Pietenpol JA, Tan AR. Triple-negative breast cancer: molecular subtypes and new targets for therapy. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet. Am Soc Clin Oncol Educ Book; 2015;e31–9. - PubMed
-
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. Nat Rev Mol Cell Biol; 2008;9:690–701. - PubMed
MeSH terms
Substances
Grants and funding
- HT9425-23-1-0166/Congressionally Directed Medical Research Programs (CDMRP)
- 22-146/Breast Cancer Research Foundation (BCRF)
- P20 GM103475/GM/NIGMS NIH HHS/United States
- R25 GM061838/GM/NIGMS NIH HHS/United States
- RO1CA248397/Center for Cancer Research (CCR)
- R16 GM149427/GM/NIGMS NIH HHS/United States
- R25GM061838/National Institute of General Medical Sciences (NIGMS)
- W81XWH2010041/Congressionally Directed Medical Research Programs (CDMRP)
- SC3 GM084824/GM/NIGMS NIH HHS/United States
- R01 CA248397/CA/NCI NIH HHS/United States
- 5P20GM103475/National Institute of General Medical Sciences (NIGMS)
- SC3GM084824/National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources
Miscellaneous

