Human milk antibodies to global pathogens reveal geographic and interindividual variations in IgA and IgG
- PMID: 39087469
- PMCID: PMC11290967
- DOI: 10.1172/JCI168789
Human milk antibodies to global pathogens reveal geographic and interindividual variations in IgA and IgG
Abstract
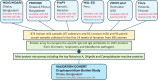
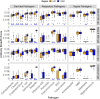
BACKGROUNDThe use of high-throughput technologies has enabled rapid advancement in the knowledge of host immune responses to pathogens. Our objective was to compare the repertoire, protection, and maternal factors associated with human milk antibodies to infectious pathogens in different economic and geographic locations.METHODSUsing multipathogen protein microarrays, 878 milk and 94 paired serum samples collected from 695 women in 5 high and low-to-middle income countries (Bangladesh, Finland, Peru, Pakistan, and the United States) were assessed for specific IgA and IgG antibodies to 1,607 proteins from 30 enteric, respiratory, and bloodborne pathogens.RESULTSThe antibody coverage across enteric and respiratory pathogens was highest in Bangladeshi and Pakistani cohorts and lowest in the U.S. and Finland. While some pathogens induced a dominant IgA response (Campylobacter, Klebsiella, Acinetobacter, Cryptosporidium, and pertussis), others elicited both IgA and IgG antibodies in milk and serum, possibly related to the invasiveness of the infection (Shigella, enteropathogenic E. coli "EPEC", Streptococcus pneumoniae, Staphylococcus aureus, and Group B Streptococcus). Besides the differences between economic regions and decreases in concentrations over time, human milk IgA and IgG antibody concentrations were lower in mothers with high BMI and higher parity, respectively. In Bangladeshi infants, a higher specific IgA concentration in human milk was associated with delayed time to rotavirus infection, implying protective properties of antirotavirus antibodies, whereas a higher IgA antibody concentration was associated with greater incidence of Campylobacter infection.CONCLUSIONThis comprehensive assessment of human milk antibody profiles may be used to guide the development of passive protection strategies against infant morbidity and mortality.FUNDINGBill and Melinda Gates Foundation grant OPP1172222 (to KMJ); Bill and Melinda Gates Foundation grant OPP1066764 funded the MDIG trial (to DER); University of Rochester CTSI and Environmental Health Sciences Center funded the Rochester Lifestyle study (to RJL); and R01 AI043596 funded PROVIDE (to WAP).
Keywords: Adaptive immunity; Immunoglobulins; Infectious disease.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

