Heterologous mRNA/MVA delivering trimeric-RBD as effective vaccination regimen against SARS-CoV-2: COVARNA Consortium
- PMID: 39087555
- PMCID: PMC11313003
- DOI: 10.1080/22221751.2024.2387906
Heterologous mRNA/MVA delivering trimeric-RBD as effective vaccination regimen against SARS-CoV-2: COVARNA Consortium
Abstract
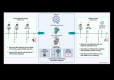
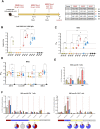
Despite the high efficiency of current SARS-CoV-2 mRNA vaccines in reducing COVID-19 morbidity and mortality, waning immunity and the emergence of resistant variants underscore the need for novel vaccination strategies. This study explores a heterologous mRNA/Modified Vaccinia virus Ankara (MVA) prime/boost regimen employing a trimeric form of the receptor binding domain (RBD) of the SARS-CoV-2 spike (S) protein compared to a homologous MVA/MVA regimen. In C57BL/6 mice, the RBD was delivered during priming via an mRNA vector encapsulated in nanoemulsions (NE) or lipid nanoparticles (LNP), followed by a booster with a replication-deficient MVA-based recombinant virus (MVA-RBD). This heterologous mRNA/MVA regimen elicited strong anti-RBD binding and neutralizing antibodies (BAbs and NAbs) against both the ancestral SARS-CoV-2 strain and different variants of concern (VoCs). Additionally, this protocol induced robust and polyfunctional RBD-specific CD4 and CD8 T cell responses, particularly in animals primed with mLNP-RBD. In K18-hACE2 transgenic mice, the LNP-RBD/MVA combination provided complete protection from morbidity and mortality following a live SARS-CoV-2 challenge compared with the partial protection observed with mNE-RBD/MVA or MVA/MVA regimens. Although the mNE-RBD/MVA regimen only protects half of the animals, it was able to induce antibodies with Fc-mediated effector functions besides NAbs. Moreover, viral replication and viral load in the respiratory tract were markedly reduced and decreased pro-inflammatory cytokine levels were observed. These results support the efficacy of heterologous mRNA/MVA vaccine combinations over homologous MVA/MVA regimen, using alternative nanocarriers that circumvent intellectual property restrictions of current mRNA vaccine formulations.
Keywords: SARS-CoV-2 vaccine; mRNA/MVA regimen; nanocarriers; trimeric-RBD; vaccine protection.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
