Cytosolic serpins act in a cytoprotective feedback loop that limits ESX-1-dependent death of Mycobacterium marinum-infected macrophages
- PMID: 39087767
- PMCID: PMC11389378
- DOI: 10.1128/mbio.00384-24
Cytosolic serpins act in a cytoprotective feedback loop that limits ESX-1-dependent death of Mycobacterium marinum-infected macrophages
Abstract
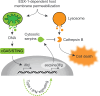
Serine protease inhibitors (serpins) constitute the largest family of protease inhibitors expressed in humans, but their role in infection remains largely unexplored. In infected macrophages, the mycobacterial ESX-1 type VII secretion system permeabilizes internal host membranes and causes leakage into the cytosol of host DNA, which induces type I interferon (IFN) production via the cyclic GMP-AMP synthase (cGAS) and stimulator of IFN genes (STING) surveillance pathway, and promotes infection in vivo. Using the Mycobacterium marinum infection model, we show that ESX-1-mediated type I IFN signaling in macrophages selectively induces the expression of serpina3f and serpina3g, two cytosolic serpins of the clade A3. The membranolytic activity of ESX-1 also caused leakage of cathepsin B into the cytosol where it promoted cell death, suggesting that the induction of type I IFN comes at the cost of lysosomal rupture and toxicity. However, the production of cytosolic serpins suppressed the protease activity of cathepsin B in this compartment and thus limited cell death, a function that was associated with increased bacterial growth in infected mice. These results suggest that cytosolic serpins act in a type I IFN-dependent cytoprotective feedback loop to counteract the inevitable toxic effect of ESX-1-mediated host membrane rupture.
Importance: The ESX-1 type VII secretion system is a key virulence determinant of pathogenic mycobacteria. The ability to permeabilize host cell membranes is critical for several ESX-1-dependent virulence traits, including phagosomal escape and induction of the type I interferon (IFN) response. We find that it comes at the cost of lysosomal leakage and subsequent host cell death. However, our results suggest that ESX-1-mediated type I IFN signaling selectively upregulates serpina3f and serpina3g and that these cytosolic serpins limit cell death caused by cathepsin B that has leaked into the cytosol, a function that is associated with increased bacterial growth in vivo. The ability to rupture host membranes is widespread among bacterial pathogens, and it will be of interest to evaluate the role of cytosolic serpins and this type I IFN-dependent cytoprotective feedback loop in the context of human infection.
Keywords: Spi2A; bacterial pathogenesis; cathepsin B; cytosolic surveillance pathways; host cell death; host-pathogen interactions; lysosome; membrane permeabilization; serpins; type I interferon; type VII secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PDR, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, et al. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107 - DOI - PMC - PubMed
-
- Lienard J, Munke K, Wulff L, Da Silva C, Vandamme J, Laschanzky K, Joeris T, Agace W, Carlsson F. 2023. Intragranuloma accumulation and inflammatory differentiation of neutrophils underlie mycobacterial ESX-1-dependent immunopathology. mBio 14:e0276422. doi: 10.1128/mbio.02764-22 - DOI - PMC - PubMed
-
- Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR. 2003. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100:12420–12425. doi: 10.1073/pnas.1635213100 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

