Efficacy of Antibiotic Regimens for Pneumonia in Young Infants Aged 0-59 Days: A Systematic Review
- PMID: 39087803
- PMCID: PMC11460314
- DOI: 10.1542/peds.2024-066588G
Efficacy of Antibiotic Regimens for Pneumonia in Young Infants Aged 0-59 Days: A Systematic Review
Abstract
Context: Pneumonia is a leading cause of death in young infants.
Objectives: To evaluate the efficacy of different antibiotic regimens to treat young infant pneumonia on critical clinical outcomes.
Data sources: MEDLINE, Embase, CINAHL, World Health Organization (WHO) Global Index Medicus, Cochrane Central Registry of Trials.
Study selection: We included randomized controlled trials of young infants aged 0 to 59 days with pneumonia (population) comparing the efficacy of antibiotic regimens (intervention) with alternate regimens or management (control) on clinical outcomes.
Data extraction: We extracted data and assessed risk of bias in duplicate. We used Grading of Recommendations, Assessment, Development, and Evaluation to assess certainty of evidence.
Limitations: Trials were heterogeneous, which precluded data pooling.
Results: Of 2601 publications screened, 10 randomized controlled trials were included. Seven trials were hospital-based (n = 869) and 3 were nonhospital-based (n = 4329). No hospital-based trials evaluated WHO-recommended first-choice regimens. One trial found the WHO-recommended second-choice antibiotic, cefotaxime, to have similar rates of treatment success as non-WHO-recommended regimens of either amoxicillin-clavulanate (RR 0.99, 95% confidence interval 0.82-1.10) or amoxicillin-clavulanate/cefotaxime (RR 1.02, 95% confidence interval 0.86-1.12). Among 3 nonhospital-based trials comparing oral amoxicillin to alternate regimens to treat isolated tachypnea among infants aged 7-59 days, there were no differences in treatment failure between amoxicillin and alternate regimens. Certainty of evidence was low or very low for all primary outcomes.
Conclusions: We found limited evidence to support the superiority of any single antibiotic regimen over alternate regimens to treat young infant pneumonia.
Conflict of interest statement
Figures
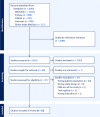

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

