Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups
- PMID: 39087959
- PMCID: PMC11443208
- DOI: 10.1158/1078-0432.CCR-24-1073
Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups
Abstract
Purpose: Elacestrant significantly prolonged progression-free survival (PFS) with manageable safety versus standard-of-care (SOC) endocrine therapy (ET) in patients with estrogen receptor-positive (ER+), HER2- metastatic breast cancer and tumors harboring estrogen receptor 1 (ESR1) mutation following ET plus a cyclin-dependent kinase 4/6 inhibitor (ET+CDK4/6i). In patients with ESR1-mutated tumors, we evaluated the efficacy and safety of elacestrant versus SOC based on prior ET+CDK4/6i duration and in clinical subgroups with prior ET+CDK4/6i ≥12 months.
Patients and methods: EMERALD, an open-label phase III trial, randomly assigned patients with ER+, HER2- metastatic breast cancer who had received 1-2 prior lines of ET, mandatory CDK4/6i, and ≤1 chemotherapy to elacestrant (345 mg daily) or SOC (aromatase inhibitor or fulvestrant). PFS was assessed across subgroups in post hoc exploratory analyses without adjustment for multiple testing.
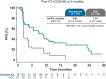
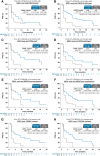
Results: In patients with ESR1-mutated tumors and prior ET+CDK4/6i ≥12 months, the median PFS for elacestrant versus SOC was 8.6 versus 1.9 months (HR, 0.41; 95% confidence interval, 0.26-0.63). In this population, the median PFS (in months) for elacestrant versus SOC was 9.1 versus 1.9 (bone metastases), 7.3 versus 1.9 (liver and/or lung metastases), 9.0 versus 1.9 (<3 metastatic sites), 10.8 versus 1.8 (≥3 metastatic sites), 5.5 versus 1.9 (PIK3 catalytic subunit α mutation), 8.6 versus 1.9 (tumor protein p53 gene mutation), 9.0 versus 1.9 (HER2-low), 9.0 versus 1.9 (ESR1D538G-mutated tumors), and 9.0 versus 1.9 (ESR1Y537S/N-mutated tumors). Subgroup safety was consistent with the overall population.
Conclusions: The duration of prior ET+CDK4/6i ≥12 months in metastatic breast cancer was associated with a clinically meaningful improvement in PFS for elacestrant compared with SOC and was consistent across all subgroups evaluated in patients with ER+, HER2-, ESR1-mutated tumors.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Bardia reports grants and personal fees from Pfizer, Genentech, Novartis, Eli Lilly and Company, Menarini, Merck, AstraZeneca, and Daiichi Sankyo during the conduct of the study. J. Cortés reports personal fees from Menarini during the conduct of the study as well as personal fees from Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, HiberCell, BioInvent, GEMoaB, Gilead, Menarini, Zymeworks, Reveal Genomics, Scorpion Therapeutics, ExpreS2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, Novartis, Eisai, Pfizer, Stemline Therapeutics, BridgeBio, BioNTech, Biocon, and Circle Pharma outside the submitted work. In addition, J. Cortés reports patents for Pharmaceutical Combinations of a PI3K Inhibitor and a Microtubule Destabilizing Agent (WO 2014/199294 A, issued) and Her2 as a Predictor of Response to Dual HER2 Blockade in the Absence of Cytotoxic Therapy (US 2019/0338368 A1, licensed); research funding (to institution) from Roche, ARIAD Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, PIQUR Therapeutics, IQVIA, and Queen Mary University of London; stock ownership in MAJ3 Capital and Leuko (relative); and travel, accommodation, and expenses from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, Gilead, Merck Sharp & Dohme, and Stemline Therapeutics. F.-C. Bidard reports personal fees from Menarini during the conduct of the study as well as personal fees from Menarini outside the submitted work. J. Garcia-Sáenz reports grants from Eli Lilly and Company, Novartis, AstraZeneca, Exact Sciences, Gilead, Adium, and Daiichi Sankyo; grants and personal fees from Menarini and Stemline; and personal fees from Jazz Pharmaceuticals outside the submitted work. P. Aftimos reports personal fees from Boehringer Ingelheim, MacroGenics, Roche, Novartis, Servier, amcure GmbH, Radius, G1 Therapeutics, Deloitte, Synthon, Gilead, Eli Lilly and Company, Menarini, and Daiichi Sankyo and nonfinancial support from Pfizer, MSD, and Amgen outside the submitted work. J. O’Shaughnessy reports personal fees from Agendia, Aptitude Health, AstraZeneca, Daiichi Sankyo, Eisai, G1 Therapeutics, Eli Lilly and Company, Loxo Oncology, Merck, Novartis, Ontada, Pfizer, Pierre Fabre, Puma Biotechnology, Roche, Samsung Bioepis, Sanofi, Seagen, Stemline Therapeutics, and Veru outside the submitted work. J. Lu reports grants from Menarini and Radius during the conduct of the study as well as grants from Radius and Eli Lilly and Company, grants and personal fees from AstraZeneca and Ambrx, and personal fees from Daiichi Sankyo and Sanofi Aventis outside the submitted work. M. Binaschi reports employment with Menarini Group. T. Wasserman reports personal fees from Menarini Group during the conduct of the study. V. Kaklamani reports personal fees from Menarini during the conduct of the study as well as personal fees from Eli Lilly and Company, Novartis, AstraZeneca, Daiichi, Gilead, TerSera, and Genentech outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in Oncology (NCCN Guidelines). Breast Cancer 2023 Mar 23. Version 4.2023. [Cited 2023 Aug 18]. Available from:https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
-
- Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. . ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475–95. - PubMed
-
- Burstein HJ. Systemic therapy for estrogen receptor-positive, HER2−negative breast cancer. N Engl J Med 2020;383:2557–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

