SGLT1 contributes to glucose-mediated exacerbation of ischemia-reperfusion injury in ex vivo rat heart
- PMID: 39088085
- PMCID: PMC11461679
- DOI: 10.1007/s00395-024-01071-z
SGLT1 contributes to glucose-mediated exacerbation of ischemia-reperfusion injury in ex vivo rat heart
Abstract
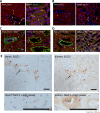
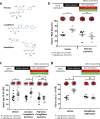
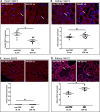
Hyperglycaemia is common during acute coronary syndromes (ACS) irrespective of diabetic status and portends excess infarct size and mortality, but the mechanisms underlying this effect are poorly understood. We hypothesized that sodium/glucose linked transporter-1 (SGLT1) might contribute to the effect of high-glucose during ACS and examined this using an ex-vivo rodent heart model of ischaemia-reperfusion injury. Langendorff-perfused rat hearts were subjected to 35 min ischemia and 2 h reperfusion, with variable glucose and reciprocal mannitol given during reperfusion in the presence of pharmacological inhibitors of SGLT1. Myocardial SGLT1 expression was determined in rat by rtPCR, RNAscope and immunohistochemistry, as well as in human by single-cell transcriptomic analysis. High glucose in non-diabetic rat heart exacerbated reperfusion injury, significantly increasing infarct size from 45 ± 3 to 65 ± 4% at 11-22 mmol/L glucose, respectively (p < 0.01), an association absent in diabetic heart (32 ± 1-37 ± 5%, p = NS). Rat heart expressed SGLT1 RNA and protein in vascular endothelium and cardiomyocytes, with similar expression found in human myocardium by single-nucleus RNA-sequencing. Rat SGLT1 expression was significantly reduced in diabetic versus non-diabetic heart (0.608 ± 0.08 compared with 1.116 ± 0.13 probe/nuclei, p < 0.01). Pharmacological inhibitors phlorizin, canagliflozin or mizagliflozoin in non-diabetic heart revealed that blockade of SGLT1 but not SGLT2, abrogated glucose-mediated excess reperfusion injury. Elevated glucose is injurious to the rat heart during reperfusion, exacerbating myocardial infarction in non-diabetic heart, whereas the diabetic heart is resistant to raised glucose, a finding which may be explained by lower myocardial SGLT1 expression. SGLT1 is expressed in vascular endothelium and cardiomyocytes and inhibiting SGLT1 abrogates excess glucose-mediated infarction. These data highlight SGLT1 as a potential clinical translational target to improve morbidity/mortality outcomes in hyperglycemic ACS patients.
Keywords: Diabetes; Hyperglycemia; Myocardial infarction; Reperfusion injury; SGLT1; SGLT2.
© 2024. The Author(s).
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Abbott GW, Tai KK, Neverisky DL, Hansler A, Hu Z, Roepke TK, Lerner DJ, Chen Q, Liu L, Zupan B, Toth M, Haynes R, Huang X, Demirbas D, Buccafusca R, Gross SS, Kanda VA, Berry GT (2014) KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. Sci Signal. 10.1126/scisignal.2005025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

