Recurrence of Non-Small Cell Lung Cancer With Visceral Pleural Invasion: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 39088196
- PMCID: PMC11295064
- DOI: 10.1001/jamaoncol.2024.2491
Recurrence of Non-Small Cell Lung Cancer With Visceral Pleural Invasion: A Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Error in Byline.JAMA Oncol. 2024 Nov 1;10(11):1598. doi: 10.1001/jamaoncol.2024.5097. JAMA Oncol. 2024. PMID: 39418063 Free PMC article. No abstract available.
Abstract
Importance: The randomized clinical trial Cancer and Leukemia Group B (CALGB) 140503 showed that for patients with clinically staged T1N0 non-small cell lung cancer (NSCLC; ≤2 cm), sublobar resections were associated with similar oncological outcomes to those after lobar resection. The association of the extent of parenchymal resection with recurrence and survival in patients with tumors pathologically upstaged to T2 based on visceral pleural invasion (VPI) is controversial.
Objective: To determine survival and recurrence rates in patients with small peripheral pT2 NSCLC (≤2 cm) that was treated by either lobar or sublobar resection in CALGB 140503.
Design, participants, and setting: CALGB 140503, a randomized multicenter noninferiority trial, included 697 patients with small peripheral NSCLC that was clinically staged as T1N0. Enrollment was from June 2007 through March 2017 at 83 participating institutions, and after a median follow-up of 7 years, the primary outcome of disease-free survival after sublobar resection was noninferior to that after lobar resection.
Intervention: Lobar or sublobar resection.
Main outcomes and measures: Survival end points were estimated by the Kaplan-Meier estimator. Hazard ratios and 95% CIs were estimated using stratified Cox proportional hazard models.
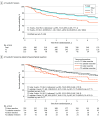
Results: Of 679 participants, 390 (57.4%) were female, and the median (range) age was 67.8 (37.8-89.7) years. Among 697 patients randomized, 566 (81.2%) had pT1 tumors (no VPI) and 113 (16.2%) had pT2 tumors (VPI). Five-year disease-free survival was 65.9% (95% CI, 61.9%-70.2%) in patients with pT1 compared with 53.3% (95% CI, 44.3%-64.1%) in patients with pT2 tumors (stratified log-rank: P = .02). Disease recurrence developed in 27.6% of patients with pT1 (locoregional only: 60 [10.8%]; distant only: 81 [14.6%]) and 41.6% of those with pT2 (locoregional only: 17 [15.0%]; distant only: 27 [23.9%]). Five-year recurrence-free survival was 73.1% (95% CI, 69.2%-77.1%) for pT1 tumors and 58.2% (95% CI, 49.2%-68.8%) for pT2 tumors (stratified log-rank: P = .01). There were no intergroup differences in disease-free or recurrence-free survival based on the extent of parenchymal resection.
Conclusions and relevance: The results of this secondary analysis suggest that compared with patients with tumors without VPI, patients who had tumors with VPI had worse disease-free and recurrence-free survival and a higher rate of local and distant disease recurrence. These high rates of recurrence were independent of the extent of parenchymal resection, and these data support the inclusion of these patients in adjuvant therapy trials.
Trial registration: ClinicalTrials.gov Identifier: NCT0049933.
Trial registration: ClinicalTrials.gov NCT00049933.
Conflict of interest statement
Figures

Comment on
-
Sublobar Resection vs Lobectomy for High-Risk Stage I Non-Small Cell Lung Carcinoma.JAMA Oncol. 2024 Sep 1;10(9):1173-1175. doi: 10.1001/jamaoncol.2024.2294. JAMA Oncol. 2024. PMID: 39088204 No abstract available.
References
-
- Saji H, Okada M, Tsuboi M, et al. ; West Japan Oncology Group and Japan Clinical Oncology Group . Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607-1617. doi: 10.1016/S0140-6736(21)02333-3 - DOI - PubMed
-
- Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Rami-Porta R, Bolejack V, Crowley J, et al. ; IASLC Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions . The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(7):990-1003. doi: 10.1097/JTO.0000000000000559 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

