Human gut microbiota-reactive DP8α Tregs prevent acute graft-versus-host disease in a CD73-dependent manner
- PMID: 39088302
- PMCID: PMC11457850
- DOI: 10.1172/jci.insight.179458
Human gut microbiota-reactive DP8α Tregs prevent acute graft-versus-host disease in a CD73-dependent manner
Abstract
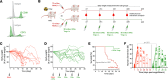
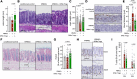
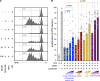
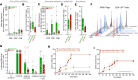
Graft-versus-host disease (GvHD) is a life-threatening complication frequently occurring following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Since gut microbiota and regulatory T cells (Tregs) are believed to play roles in GvHD prevention, we investigated whether DP8α Tregs, which we have previously described to harbor a T cell receptor specificity for the gut commensal Faecalibacterium prausnitzii, could protect against GvHD, thereby linking the microbiota and its effect on GvHD. We observed a decrease in CD73+ DP8α Treg frequency in allo-HSCT patients 1 month after transplantation, which was associated with acute GvHD (aGvHD) development at 1 month after transplantation, as compared with aGvHD-free patients, without being correlated to hematological disease relapse. Importantly, CD73 activity was shown to be critical for DP8α Treg suppressive function. Moreover, the frequency of host-reactive DP8α Tregs was also lower in aGvHD patients, as compared with aGvHD-free patients, which could embody a protective mechanism responsible for the maintenance of this cell subset in GvHD-free patients. We also showed that human DP8α Tregs protected mice against xenogeneic GvHD through limiting deleterious inflammation and preserving gut integrity. Altogether, these results demonstrated that human DP8α Tregs mediate aGvHD prevention in a CD73-dependent manner, likely through host reactivity, advocating for the use of these cells for the development of innovative therapeutic strategies to preclude aGvHD-related inflammation.
Keywords: Immunology; Immunotherapy; T cells; Translation; Transplantation.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

