Loss of the long form of Plod2 phenocopies contractures of Bruck syndrome-osteogenesis imperfecta
- PMID: 39088537
- PMCID: PMC11371901
- DOI: 10.1093/jbmr/zjae124
Loss of the long form of Plod2 phenocopies contractures of Bruck syndrome-osteogenesis imperfecta
Abstract
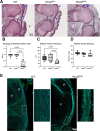
Bruck syndrome is an autosomal recessive form of osteogenesis imperfecta caused by biallelic variants in PLOD2 or FKBP10 and is characterized by joint contractures, bone fragility, short stature, and scoliosis. PLOD2 encodes LH2, which hydroxylates type I collagen telopeptide lysines, a critical step for collagen crosslinking. The Plod2 global knockout mouse model is limited by early embryonic lethality, and thus, the role of PLOD2 in skeletogenesis is not well understood. We generated a novel Plod2 mouse line modeling a variant identified in two unrelated individuals with Bruck syndrome: PLOD2 c.1559dupC, predicting a frameshift and loss of the long isoform LH2b. In the mouse, the duplication led to loss of LH2b mRNA as well as significantly reduced total LH2 protein. This model, Plod2fs/fs, survived up to E18.5 although in non-Mendelian genotype frequencies. The homozygous frameshift model recapitulated the joint contractures seen in Bruck syndrome and had indications of absent type I collagen telopeptide lysine hydroxylation in bone. Genetically labeling tendons with Scleraxis-GFP in Plod2fs/fs mice revealed the loss of extensor tendons in the forelimb by E18.5, and developmental studies showed extensor tendons developed through E14.5 but were absent starting at E16.5. Second harmonic generation showed abnormal tendon type I collagen fiber organization, suggesting structurally abnormal tendons. Characterization of the skeleton by μCT and Raman spectroscopy showed normal bone mineralization levels. This work highlights the importance of properly crosslinked type I collagen in tendon and bone, providing a promising new mouse model to further our understanding of Bruck syndrome.
Keywords: Bruck syndrome; LH2; PLOD2; bone; collagen crosslinking; hydroxylysine; mouse model; osteogenesis imperfecta; tendon.
Plain language summary
Bruck syndrome is a rare disease where individuals have brittle bone as well as contracted or stiff joints. Mutations in two genes are associated with Bruck syndrome and, in this work, we focus on PLOD2. Mice without Plod2 die at an early embryonic stage, before they have a chance to fully develop. In this work, we created a mouse with a PLOD2 mutation seen in people with Bruck syndrome. Some of these new Bruck syndrome model mice survived to a later gestational age, but all died at birth. The Bruck syndrome mice were small and had contracted joints. We found that they were missing tendons in their arms and had structurally abnormal tendons in their knees. Bone mineralization was normal, but there were indications that the modifications needed for normal type I collagen structure were absent. Overall, this is an advantageous new mouse model of Bruck syndrome that can be used to study this rare disease and highlights the importance of Plod2 in tendon.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

