Trastuzumab Deruxtecan in Advanced Solid Tumors With Human Epidermal Growth Factor Receptor 2 Amplification Identified by Plasma Cell-Free DNA Testing: A Multicenter, Single-Arm, Phase II Basket Trial
- PMID: 39088783
- PMCID: PMC11542975
- DOI: 10.1200/JCO.23.02626
Trastuzumab Deruxtecan in Advanced Solid Tumors With Human Epidermal Growth Factor Receptor 2 Amplification Identified by Plasma Cell-Free DNA Testing: A Multicenter, Single-Arm, Phase II Basket Trial
Abstract
Purpose: HERALD/EPOC1806 was conducted as a multicenter phase II trial assessing trastuzumab deruxtecan (T-DXd) therapy for patients with human epidermal growth factor receptor 2 (HER2)-amplified progressive stage solid tumors detected by cell-free DNA (cfDNA) testing.
Patients and methods: Patients exhibited advanced solid tumors with HER2 amplification that was identified via next-generation sequencing of cfDNA testing, without the requirement for immunohistochemical HER2 testing. The studied group was administered T-DXd at 5.4 mg/kg once every 3 weeks until onset of disease progression or intolerable toxicity.
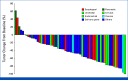
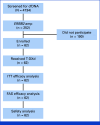
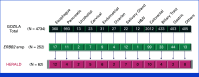
Results: Overall, 4,734 patients underwent cfDNA testing from December 2019 to January 2022, and 252 demonstrated HER2 amplification. Finally, the study included 62 patients with 16 cancer types with a median baseline plasma HER2 copy number (CN) of 8.55 (range, 2.4-73.9). Confirmed overall response rate (ORR) by investigator assessment was 56.5% (95% CI, 43.3 to 69.0), thus showing a value beyond the 5% threshold. Responses were evaluated for 13 cancer types, including KRAS-mutant colorectal (1/3), PIK3CA-mutant endometrial (5/6), and tissue HER2-negative gastric (1/2) cancers. Plasma HER2 CN above versus below the baseline median value did not differ for impact response; however, clearance of HER2 amplification in cfDNA on cycle 2 day 1 had higher response values compared with persistence. Median progression-free survival and response duration were 7.0 (95% CI, 4.9 to 9.7) and 8.8 (95% CI, 5.8 to 11.2) months, respectively, with the majority of complications being mild to moderate. Interstitial lung diseases were identified in 16 (26%) patients, including 14 patients with grade 1 disease, one patient with grade 2 disease, and one patient with grade 3 disease.
Conclusion: T-DXd treatment demonstrated high ORR with durable response in patients with advanced HER2-amplified solid tumors determined with cfDNA testing.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures




Comment in
-
Trastuzumab deruxtecan: expanding the role of HER2-targeted therapy in solid tumors.Transl Cancer Res. 2025 Jun 30;14(6):3286-3291. doi: 10.21037/tcr-2025-400. Epub 2025 Jun 12. Transl Cancer Res. 2025. PMID: 40687244 Free PMC article. No abstract available.
References
-
- Strickler JH, Cercek A, Siena S, et al. : Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol 24:496-508, 2023 - PubMed
-
- Oh DY, Bang YJ: HER2-targeted therapies—A role beyond breast cancer. Nat Rev Clin Oncol 17:33-48, 2020 - PubMed
-
- Dumbrava EEI, Balaji K, Raghav K, et al. : Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol 10.1200/PO.18.00345 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

