The choroid plexus synergizes with immune cells during neuroinflammation
- PMID: 39089253
- PMCID: PMC11458255
- DOI: 10.1016/j.cell.2024.07.002
The choroid plexus synergizes with immune cells during neuroinflammation
Abstract
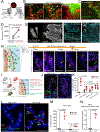
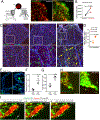
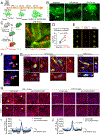
The choroid plexus (ChP) is a vital brain barrier and source of cerebrospinal fluid (CSF). Here, we use longitudinal two-photon imaging in awake mice and single-cell transcriptomics to elucidate the mechanisms of ChP regulation of brain inflammation. We used intracerebroventricular injections of lipopolysaccharides (LPS) to model meningitis in mice and observed that neutrophils and monocytes accumulated in the ChP stroma and surged across the epithelial barrier into the CSF. Bi-directional recruitment of monocytes from the periphery and, unexpectedly, macrophages from the CSF to the ChP helped eliminate neutrophils and repair the barrier. Transcriptomic analyses detailed the molecular steps accompanying this process and revealed that ChP epithelial cells transiently specialize to nurture immune cells, coordinating their recruitment, survival, and differentiation as well as regulation of the tight junctions that control the permeability of the ChP brain barrier. Collectively, we provide a mechanistic understanding and a comprehensive roadmap of neuroinflammation at the ChP brain barrier.
Keywords: adhesion molecules; bacterial infection; blood-CSF barrier; choroid plexus; colony-stimulating factor 1; epiplexus macrophages; epithelial cells; immune recruitment; neuroinflammation.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.O.-M. reports compensation for consulting services with Cellarity, Tessel Biosciences, and Radera Biotherapeutics. J.C. has been an employee of Dyne Therapeutics since April 2021. Her contributions to this manuscript were made when she was employed by Boston Children’s Hospital.
Figures



References
MeSH terms
Substances
Grants and funding
- R01 NS088566/NS/NINDS NIH HHS/United States
- R01 NS129823/NS/NINDS NIH HHS/United States
- R01 AI168005/AI/NIAID NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- R01 HL162642/HL/NHLBI NIH HHS/United States
- DP1 AT010971/AT/NCCIH NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- T32 GM144273/GM/NIGMS NIH HHS/United States
- T32 GM008313/GM/NIGMS NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- T32 NS007473/NS/NINDS NIH HHS/United States
- RF1 DA048790/DA/NIDA NIH HHS/United States
- F32 NS136267/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

