Lack of prion transmission barrier in human PrP transgenic Drosophila
- PMID: 39089583
- PMCID: PMC11386037
- DOI: 10.1016/j.jbc.2024.107617
Lack of prion transmission barrier in human PrP transgenic Drosophila
Abstract
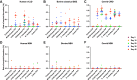
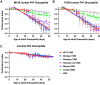
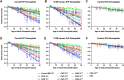
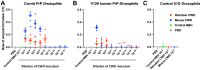
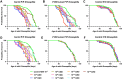
While animal prion diseases are a threat to human health, their zoonotic potential is generally inefficient because of interspecies prion transmission barriers. New animal models are required to provide an understanding of these prion transmission barriers and to assess the zoonotic potential of animal prion diseases. To address this goal, we generated Drosophila transgenic for human or nonhuman primate prion protein (PrP) and determined their susceptibility to known pathogenic prion diseases, namely varient Creutzfeldt-Jakob disease (vCJD) and classical bovine spongiform encephalopathy (BSE), and that with unknown pathogenic potential, namely chronic wasting disease (CWD). Adult Drosophila transgenic for M129 or V129 human PrP or nonhuman primate PrP developed a neurotoxic phenotype and showed an accelerated loss of survival after exposure to vCJD, classical BSE, or CWD prions at the larval stage. vCJD prion strain identity was retained after passage in both M129 and V129 human PrP Drosophila. All of the primate PrP fly lines accumulated prion seeding activity and concomitantly developed a neurotoxic phenotype, generally including accelerated loss of survival, after exposure to CWD prions derived from different cervid species, including North American white-tailed deer and muntjac, and European reindeer and moose. These novel studies show that primate PrP transgenic Drosophila lack known prion transmission barriers since, in mammalian hosts, V129 human PrP is associated with severe resistance to classical BSE prions, while both human and cynomolgus macaque PrP are associated with resistance to CWD prions. Significantly, our data suggest that interspecies differences in the amino acid sequence of PrP may not be a principal determinant of the prion transmission barrier.
Keywords: BSE; Drosophila; chronic wasting disease; human; neurodegenerative disease; nonhuman primate; prion; transgenic; vCJD; zoonotic.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Prusiner S.B. second edition. Cold Spring Harbor Laboratory Press; New York: 2004. Prion Biology and Diseases.
-
- Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
-
- Castilla J., Saa P., Hetz C., Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
-
- Legname G., Baskakov I.V., Nguyen H.O., Riesner D., Cohen F.E., DeArmond S.J., et al. Synthetic mammalian prions. Science. 2004;305:673–676. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

