Interpenetrating Polymer Network Capturing FRET-Sensitive Polymers Available for an Enzyme Assay
- PMID: 39089682
- PMCID: PMC11323839
- DOI: 10.1021/acs.biomac.4c00622
Interpenetrating Polymer Network Capturing FRET-Sensitive Polymers Available for an Enzyme Assay
Abstract
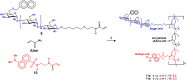
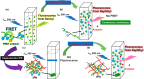
Fluorogenic glycomonomers have been used for biological evaluations, and water-soluble and Förster resonance energy transfer (FRET)-sensitive glycopolymers have also been reported. A FRET-sensitive polymer was conveniently prepared from a fluorogenic donor monomer and a fluorogenic acceptor monomer by means of simple radical polymerization in high yield. Continuous fluorospectroscopic monitoring of the polymer in the presence of an enzyme was performed, and the results showed the possible application of the FRET-sensitive glycopolymer for practical use. In addition to the use of aqueous solution phase, the water-soluble and FRET-sensitive glycopolymer was completely captured into an interpenetrating polymer network (IPN) by means of radical polymerization with a combination of acrylamide and bis-acrylamide as used for the cross-linking reagent system. The IPN including the FRET-sensitive glycopolymer was allowed to react with amylases in an aqueous buffer solution at 37 °C, and the enzymatic reaction was continuously and conveniently monitored by means of fluorometric spectroscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Viable E. coli O157:H7 detection based on Föster resonance energy transfer (FRET) system using FITC and TRITC conjugates as molecular probes.MethodsX. 2025 Jun 6;14:103406. doi: 10.1016/j.mex.2025.103406. eCollection 2025 Jun. MethodsX. 2025. PMID: 40584166 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Early insulin fibril detection: Insulin fibril research and TR structural transition detection with FRET-Probe.Anal Chim Acta. 2025 Oct 1;1369:344366. doi: 10.1016/j.aca.2025.344366. Epub 2025 Jun 25. Anal Chim Acta. 2025. PMID: 40701730
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
References
-
- Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 437, 55–75. 10.1002/andp.19484370105. - DOI
-
- Stryer L.; Haugland R. P. Energy transfer: a spectroscopic ruler. Proc. Natl. Acad. Sci. U.S.A. 1967, 58, 719–726. 10.1073/pnas.58.2.719. - DOI - PMC - PubMed
- Stryer L. Fluorescence Energy-Transfer as a Spectroscopic Ruler. Annu. Rev. Biochem. 1978, 47, 819–846. 10.1146/annurev.bi.47.070178.004131. - DOI - PubMed
-
- Szollosi J.; Damjanovich S.; Matyus L. Application of Fluorescence Resonance Energy Transfer in the Clinical Laboratory: Routine and Research. Cytometry 1998, 34, 159–179. 10.1002/(SICI)1097-0320(19980815)34:4<159::AID-CYTO1>3.0.CO;2-B. - DOI - PubMed
- Rice K. G. Application of Fluorescence Resonance Energy Transfer to Analyze Carbohydrates. Anal. Biochem. 2001, 297, 117–122. 10.1006/abio.2001.5310. - DOI - PubMed
- Didenko V. V. DNA Probes using Fluorescence Resonance Energy Transfer (FRET): Designs and Applications. Biotechniques 2001, 31, 1106–1121. 10.2144/01315rv02. - DOI - PMC - PubMed
- Jares-Erijman E. A.; Jovin T. M. FRET Imaging. Nat. Biotechnol. 2003, 21, 1387–1395. 10.1038/nbt896. - DOI - PubMed
- Sapsford K. E.; Berti L.; Medintz I. L. Materials for Fluorescence Resonance Energy Transfer Analysis: Beyond Traditional Donor-Acceptor Combinations. Angew. Chem., Int. Ed. 2006, 45, 4562–4589. 10.1002/anie.200503873. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

