Efficacy and Safety of Lurasidone vs. Quetiapine XR in Acutely Psychotic Patients With Schizophrenia in Korea: A Randomized, Double-Blind, Active-Controlled Trial
- PMID: 39089702
- PMCID: PMC11298266
- DOI: 10.30773/pi.2024.0052
Efficacy and Safety of Lurasidone vs. Quetiapine XR in Acutely Psychotic Patients With Schizophrenia in Korea: A Randomized, Double-Blind, Active-Controlled Trial
Abstract
Objective: This study was performed to evaluate the efficacy and safety of lurasidone (160 mg/day) compared to quetiapine XR (QXR; 600 mg/day) in the treatment of acutely psychotic patients with schizophrenia.
Methods: Patients were randomly assigned to 6 weeks of double-blind treatment with lurasidone 160 mg/day (n=105) or QXR 600 mg/day (n=105). Primary efficacy measure was the change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score and Clinical Global Impressions severity (CGI-S) score. Adverse events, body measurements, and laboratory parameters were assessed.
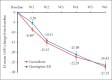
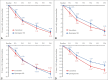
Results: Lurasidone demonstrated non-inferiority to QXR on the PANSS total score. Adjusted mean±standard error change at week 6 on the PANSS total score was -26.42±2.02 and -27.33±2.01 in the lurasidone and QXR group, respectively. The mean difference score was -0.91 (95% confidence interval -6.35-4.53). The lurasidone group showed a greater reduction in PANSS total and negative subscale on week 1 and a greater reduction in end-point CGI-S score compared to the QXR group. Body weight, body mass index, and waist circumference in the lurasidone group were reduced, with significantly lower mean change compared to QXR. Endpoint changes in glucose, cholesterol, triglycerides, and low-density lipoprotein levels were also significantly lower. The most common adverse drug reactions with lurasidone were akathisia and nausea.
Conclusion: Lurasidone 160 mg/day was found to be non-inferior to QXR 600 mg/day in the treatment of schizophrenia with comparable efficacy and tolerability. Adverse effects of lurasidone were generally tolerable, and beneficial effects on metabolic parameters can be expected.
Keywords: Efficacy; Lurasidone; Randomized clinical trial; Safety.
Conflict of interest statement
Sung Won Roh, Jong-Woo Paik, and Euitae Kim a contributing editor of the
Figures
References
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. - PubMed
-
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. - PubMed
-
- Pompili M, Verzura C, Trovini G, Buscajoni A, Falcone G, Naim S, et al. Lurasidone: efficacy and safety in the treatment of psychotic and mood disorders. Expert Opin Drug Saf. 2018;17:197–205. - PubMed
-
- Murai T, Nakako T, Ikeda K, Ikejiri M, Ishiyama T, Taiji M. Lack of dopamine D4 receptor affinity contributes to the procognitive effect of lurasidone. Behav Brain Res. 2014;261:26–30. - PubMed
-
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334:171–181. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

