Blockade of glucose-6-phosphate dehydrogenase induces immunogenic cell death and accelerates immunotherapy
- PMID: 39089738
- PMCID: PMC11293396
- DOI: 10.1136/jitc-2023-008441
Blockade of glucose-6-phosphate dehydrogenase induces immunogenic cell death and accelerates immunotherapy
Abstract
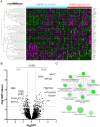
Background: Enhanced glucose metabolism has been reported in many cancers. Glucose-6-phosphate dehydrogenase (G6PD) is a rate-limiting enzyme involved in the pentose phosphate pathway, which maintains NADPH levels and protects cells from oxidative damage. We recently found that low G6PD expression correlates with active tumor immunity. However, the mechanism involving G6PD and tumor immunity remained unclear.
Methods: We conducted in vitro studies using G6PD-knocked down malignant melanoma cells, pathway analysis using the GEO dataset, in vivo studies in combination with immune checkpoint inhibitors (ICIs) using a mouse melanoma model, and prognostic analysis in 42 melanoma patients and 30 lung cancer patients who were treated with ICIs.
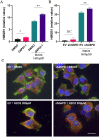
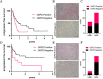
Results: Inhibition of G6PD, both chemically and genetically, has been shown to decrease the production of NADPH and reduce their oxidative stress tolerance. This leads to cell death, which is accompanied by the release of high mobility group box 1 and the translocation of calreticulin to the plasma membrane. These findings suggested that inhibiting G6PD can induce immunogenic cell death. In experiments with C57BL/6 mice transplanted with G6PD-knockdown B16 melanoma cells and treated with anti-PD-L1 antibody, a significant reduction in tumor size was observed. Interestingly, inhibiting G6PD in only a part of the lesions increased the sensitivity of other lesions to ICI. Additionally, out of 42 melanoma patients and 30 lung cancer patients treated with ICIs, those with low G6PD expression had a better prognosis than those with high G6PD expression (p=0.0473; melanoma, p=0.0287; lung cancer).
Conclusion: G6PD inhibition is a potent therapeutic strategy that triggers immunogenic cell death in tumors, significantly augmenting the efficacy of immunotherapies.
Keywords: Immune Checkpoint Inhibitors; Immunotherapy; Melanoma; Non-Small Cell Lung Cancer.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Nagoya City University is submitting patents for the G6PD test methods. MN invented the G6PD test method.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
