Myelographic Techniques for the Localization of CSF-Venous Fistulas: Updates in 2024
- PMID: 39089875
- PMCID: PMC11449000
- DOI: 10.3174/ajnr.A8299
Myelographic Techniques for the Localization of CSF-Venous Fistulas: Updates in 2024
Abstract
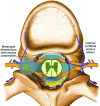
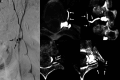
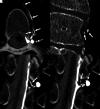
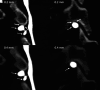
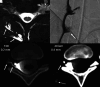
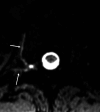
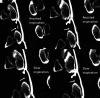
CSF-venous fistulas (CVFs) are a common cause of spontaneous intracranial hypotension. Despite their relatively frequent occurrence, they can be exceedingly difficult to detect on imaging. Since the initial description of CVFs in 2014, the recognition and diagnosis of this type of CSF leak has continually increased. As a result of multi-institutional efforts, a wide spectrum of imaging modalities and specialized techniques for CVF detection is now available. It is important for radiologists to be familiar with the multitude of available techniques, because each has unique advantages and drawbacks. In this article, we review the spectrum of imaging modalities available for the detection of CVFs, explain the advantages and disadvantages of each, provide typical imaging examples, and discuss provocative maneuvers that may improve the conspicuity of CVFs. Discussed modalities include conventional CT myelography, dynamic myelography, digital subtraction myelography, conebeam CT myelography, decubitus CT myelography by using conventional energy-integrating detector scanners, decubitus photon counting CT myelography, and intrathecal gadolinium MR myelography. Additional topics to be discussed include optimal patient positioning, respiratory techniques, and intrathecal pressure augmentation.
© 2024 by American Journal of Neuroradiology.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
