A male mouse model for metabolic dysfunction-associated steatotic liver disease and hepatocellular carcinoma
- PMID: 39090079
- PMCID: PMC11294468
- DOI: 10.1038/s41467-024-50660-y
A male mouse model for metabolic dysfunction-associated steatotic liver disease and hepatocellular carcinoma
Abstract
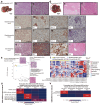
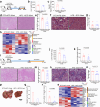
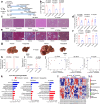
The lack of an appropriate preclinical model of metabolic dysfunction-associated steatotic liver disease (MASLD) that recapitulates the whole disease spectrum impedes exploration of disease pathophysiology and the development of effective treatment strategies. Here, we develop a mouse model (Streptozotocin with high-fat diet, STZ + HFD) that gradually develops fatty liver, metabolic dysfunction-associated steatohepatitis (MASH), hepatic fibrosis, and hepatocellular carcinoma (HCC) in the context of metabolic dysfunction. The hepatic transcriptomic features of STZ + HFD mice closely reflect those of patients with obesity accompanying type 2 diabetes mellitus, MASH, and MASLD-related HCC. Dietary changes and tirzepatide administration alleviate MASH, hepatic fibrosis, and hepatic tumorigenesis in STZ + HFD mice. In conclusion, a murine model recapitulating the main histopathologic, transcriptomic, and metabolic alterations observed in MASLD patients is successfully established.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

