Systemic mechanisms of necrotic cell debris clearance
- PMID: 39090111
- PMCID: PMC11294570
- DOI: 10.1038/s41419-024-06947-5
Systemic mechanisms of necrotic cell debris clearance
Abstract
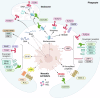
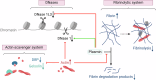
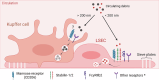
Necrosis is an overarching term that describes cell death modalities caused by (extreme) adverse conditions in which cells lose structural integrity. A guaranteed consequence of necrosis is the production of necrotic cell remnants, or debris. Necrotic cell debris is a strong trigger of inflammation, and although inflammatory responses are required for tissue healing, necrotic debris may lead to uncontrolled immune responses and collateral damage. Besides local phagocytosis by recruited leukocytes, there is accumulating evidence that extracellular mechanisms are also involved in necrotic debris clearance. In this review, we focused on systemic clearance mechanisms present in the bloodstream and vasculature that often cooperate to drive the clearance of cell debris. We reviewed the contribution and cooperation of extracellular DNases, the actin-scavenger system, the fibrinolytic system and reticuloendothelial cells in performing clearance of necrotic debris. Moreover, associations of the (mis)functioning of these clearance systems with a variety of diseases were provided, illustrating the importance of the mechanisms of clearance of dead cells in the organism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

