Anti-SARS-CoV-2 antibody dynamics after primary vaccination with two-dose inactivated whole-virus vaccine, heterologous mRNA-1273 vaccine booster, and Omicron breakthrough infection in Indonesian health care workers
- PMID: 39090537
- PMCID: PMC11292869
- DOI: 10.1186/s12879-024-09644-y
Anti-SARS-CoV-2 antibody dynamics after primary vaccination with two-dose inactivated whole-virus vaccine, heterologous mRNA-1273 vaccine booster, and Omicron breakthrough infection in Indonesian health care workers
Abstract
Background: Data on the dynamics and persistence of humoral immunity against SARS-CoV-2 after primary vaccination with two-dose inactivated vaccine (CoronaVac) are limited. This study evaluated the sequential effects of prior infection, heterologous boosting with mRNA-1273 (Moderna), and the occurrence of Omicron vaccine-breakthrough infection (VBI) thereafter.
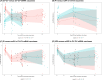
Methods: We evaluated anti-spike IgG (Abbott) and neutralising (cPASS/GenScript) antibody (nAb) titers up to one year after mRNA-1273 boost in two-dose-CoronaVac-primed Indonesian healthcare workers (August 2021-August 2022). We used linear mixed modeling to estimate the rate of change in antibody levels, and logistic regression to examine associations between antibody levels and VBI.
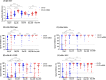
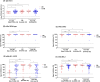
Results: Of 138 participants, 52 (37.7%) had a prior infection and 78 (56.5%) received an mRNA-1273 booster. After two-dose CoronaVac, antibody titers had significantly declined within 180 days, irrespective of prior infection. After mRNA-1273 booster, anti-spike IgG (1.47% decline/day) and Omicron B.1.1.529/BA.2 nAbs declined between day 28-90, and IgG titers plateaued between day 90-360. During the BA.1/BA.2 wave (February-March 2022), 34.6% (27/78) of individuals experienced a VBI (median 181 days after mRNA-1273), although none developed severe illness. VBI was associated with low pre-VBI anti-spike IgG and B.1.1.529/BA.2 nAbs, which were restored post-VBI.
Conclusions: mRNA-1273 booster after two-dose CoronaVac did not prevent BA.1/BA.2 VBI. Periodic vaccine boosters may be warranted against emerging SARS-CoV-2 variants.
Keywords: COVID-19; CoronaVac; Humoral immunity; SARS-CoV-2; mRNA-1273.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–9. 10.1016/S0140-6736(22)00094-0. 10.1016/S0140-6736(22)00094-0 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

