Immunogenicity and safety of an Entamoeba histolytica adjuvanted protein vaccine candidate (LecA+GLA-3M-052 liposomes) in rhesus macaques
- PMID: 39090779
- PMCID: PMC11296537
- DOI: 10.1080/21645515.2024.2374147
Immunogenicity and safety of an Entamoeba histolytica adjuvanted protein vaccine candidate (LecA+GLA-3M-052 liposomes) in rhesus macaques
Abstract
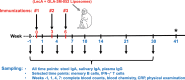
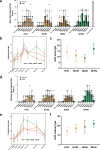
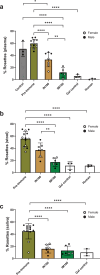
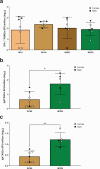
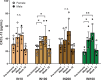
Entamoeba histolytica, the causative agent of amebiasis, is one of the top three parasitic causes of mortality worldwide. However, no vaccine exists against amebiasis. Using a lead candidate vaccine containing the LecA fragment of Gal-lectin and GLA-3M-052 liposome adjuvant, we immunized rhesus macaques via intranasal or intramuscular routes. The vaccine elicited high-avidity functional humoral responses as seen by the inhibition of amebic attachment to mammalian target cells by plasma and stool antibodies. Importantly, antigen-specific IFN-γ-secreting peripheral blood mononuclear cells (PBMCs) and IgG/IgA memory B cells (BMEM) were detected in immunized animals. Furthermore, antigen-specific antibody and cellular responses were maintained for at least 8 months after the final immunization as observed by robust LecA-specific BMEM as well as IFN-γ+ PBMC responses. Overall, both intranasal and intramuscular immunizations elicited a durable and functional response in systemic and mucosal compartments, which supports advancing the LecA+GLA-3M-052 liposome vaccine candidate to clinical testing.
Keywords: Entamoeba histolytica; amebiasis; intranasal delivery; mucosal adjuvant; rhesus macaque.
Conflict of interest statement
The authors declare no Competing Non-Financial Interests but the following Competing Financial Interests. MAT is a contract worker for 3M, and 3M-052 is an asset of 3M Healthcare. WAP is a consultant for TechLab, Inc. and in addition receives royalties for amebiasis diagnostics that are donated in their entirety to the American Society of Tropical Medicine and Hygiene. KP is an employee of TechLab, Inc., and amebiasis diagnostics are an asset of TechLab’s. MMA, WAP, and CBF are inventors on patents and/or patent applications involving the vaccine formulation represented in this article. All other authors declare no competing interests.
Figures

References
-
- WHO/PAHO/UNESCO report . A consultation with experts on amoebiasis. Epidemiol Bull. Mexico City, Mexico; 1997. 1997 Jan 28–29; 18:13–13. - PubMed
-
- Tellevik MG, Moyo SJ, Blomberg B, Hjøllo T, Maselle SY, Langeland N, Hanevik K, Jex AR. Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLOS Negl Trop Dis. 2015;9(10):e0004125. doi: 10.1371/journal.pntd.0004125. - DOI - PMC - PubMed
-
- Mondal D, Petri WA, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006;100(11):1032–8. doi: 10.1016/j.trstmh.2005.12.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
